Created by Boniventure Yohana Salumu
Phone no;0757388430 or 0688836550 or 0789434527
FORM ONE CHEMISTRY NOTES
CHAPTER ONE:INTRODUCTION TO CHEMISTRY AND LABORATORY
TOPIC 1: INTRODUCTION TO CHEMISTRY
TOPIC 2: LABORATORY TECHNIQUES AND SAFETY
Harmful substances are those that can impair your health or make you fall sick. They do not normally kill instantly but have detrimental effects following a long exposure to them. These chemicals do not kill immediately. However, care must be taken when handling or dealing with them. Irritating substances cause pains when in contact with the body. They are dangerous to health when in contact with the body surface for a long period of time.
OR
Definition; Chemistry is a branch of science that deals with the study of nature, properties and composition of matter.
TOPIC 1: INTRODUCTION TO CHEMISTRY
Introduction To Chemistry
When you were in primary school, you used to learn science as a single subject. At this level of study, the subject will be broken up into three related subjects, namely Chemistry, Biology and Physics. The three subjects are closely related. You will require the knowledge of one subject to study the other. For example, you will apply the knowledge of chemistry to study different chemical reactions that take place in the body for studying biology of the human body, etc. Likewise as a biologist, you will need the knowledge of physics to study movements of different limbs of the body, etc. Therefore, these few examples show how the three subjects are interdependent. Chemistry is usually studied along with other related subjects such as biology, physics, earth sciences and mathematics. A person studying science is called a scientist. A scientist specialized in the study of chemistry is called a chemist.
TOPIC 2: LABORATORY TECHNIQUES AND SAFETY
A laboratory is a room or building specially designed for conducting various scientific experiments. An appropriate school laboratory has the following features:
- a room with enough space for carrying out scientific experiments;
- a store for keeping laboratory apparatus, chemicals and reagents;
- an office for laboratory technician to sit in and design scientific experiments;
- enough ventilation to let in fresh air and light;
- wide doors and several exits for emergency evacuation in case of an accident; and
- a wide table in front of the laboratory room, fitted with sinks for experiment demonstrations by the teacher or technician.
OR
Definition; Chemistry is a branch of science that deals with the study of nature, properties and composition of matter.
Matter can be defined as anything that has weight or mass and can occupy space.
Therefore, in chemistry we study materials that make up the earth and universe. These range from living to non-living materials. We apply the knowledge of chemistry to study the
composition, behaviour and nature of materials around us. This study enables us to make the best
use of these materials to improve our welfare.
APLICATION OF CHEMISTRY
Materials/Objects Made by Application of Chemistry
Mention materials objects made by application of chemistry
Chemistry is such an important subject that it is applied in other fields such as agriculture,
manufacturing, medicine, processing and food industries, education, cosmetics and home care
industries, etc. All these industries are responsible for the production of materials that we need to
support and hence improve our lives. Materials made by the application of chemistry knowledge
include soap, chalk, shoes, clothes, petroleum products, alcoholic and non-alcoholic beverages,
cosmetics, drugs, and many others. Can you mention some of the materials made by the
application of chemistry knowledge?
This, therefore, means that chemistry is applied in factories, homes, hospitals, pharmacies,
research centers, higher learning institutions, etc.
Many products made by the application of chemistry in industry are all around us. Some of these
materials are summarized in the table:
Field where applied Examples of products
3.Some products made by application of chemistry
The importance of chemistry in life
Areas Where Chemistry is Applied
Mention areas where chemistry is applied
In everyday life, we need different substances to meet our basic human needs like food, shelter,
clothing, comfort and health. Application of chemical knowledge enables the production of
different materials and products that we need to live better.
Examples of these materials, as mentioned early, are (paraffin), sugar, common salt, soft drinks,
medical drugs (medicines), toothpaste and plastics. Others are spirits, wines, shoe polishes,
cement, baking soda, petrol, diesel and cosmetics (soaps, body oils and lotions, body and hair
creams, etc)
All these materials, among others, are made by applying chemical processes. They are needed
for a better living. Can you mention more materials made through chemistry knowledge?
Medicine Drugs, vaccines, nutritional supplements
Agriculture
Agri-chemicals ( fertilizers, pesticides, herbicides, acaricides), animal drugs and vaccines,
animal feed and supplements
Manufacturing industry Vehicles, cement, plastics, chemicals, paints, iron sheets, vanishes, glue
Food and beverage industry Soft and alcoholic drinks, baked food, canned food, spices, cooking oil, salt
Home care and cosmetics
industry Cosmetics, detergents, toothpaste, shoe polish, insecticides, antiseptics, disinfectants
Transport Fuels, lubricants, oil, grease, tar, coolants, tyres
Textile industry Clothes, dyes, bleaches, wax, threads
Leather industry Shoes, handbags, belts, leather articles
4.Some materials made by application of chemistry
Nature is made of materials that may be useless, less useful and even harmful. There are also
things that are very useful to our lives. Through chemistry, we are able to transform (change)
various materials chemically or physically into forms or products that are more useful to man
For example, most laboratory chemicals you use at school are prepared from minerals that are
mined from the rocks in the earth.
Laboratory chemicals
Man cannot use most substances unless they are transformed into products that are more useful.
Limestone lying idle in earth is useless until it undergoes deliberate physical and compositional
transformation into cement. The cement is used for construction of buildings, roads, bridges and
many different structures.
5.We also need to change different mineral ores through a number of processes into useful
substances such as steel, aluminium, tin, etc. Man has learned how to change harmful substances
into useful products since the long ago.
Common salt may be made from twohazardous substances–hydrochloric acid and sodium
hydroxide.
Chemistry is all around us. We often use chemical products and engage ourselves in chemical
processes more than we can tell. Look at the picture below.
This is an example of a chemical activity in which we can engage ourselves without knowing.
A woman washing clothes
6.Many items we use at school, home and industry are made by applying chemical processes. The
soap we use to wash our clothes and clean our bodies is made from animal fat and an alkali.
Many items are made from plastic. Many kinds of plastics are made from crude oil. What items
are made from plastics in your home? Soft drink bottles are made from glass. The major
component of glass is sand. Glass is made by mixing sand with metal oxides in a furnace at high
temperatures. Some clothing is made from natural fibers such as cotton or silk.
Other fabrics like polyester and nylon are made from chemicals found in coal and crude oil.
What are your clothes made of?
Clothes made from cotton fibres
Man has used medicines extracted from plants and animals since the beginning of time. For
example, cinchona tree contains quinine, which has a bitter taste. Quinine was and is still used
for treatment of malaria. Penicillin is extracted from a fungus called penicillin. Nowadays, it is
possible to make chemicals that have the same effects as naturally occurring drugs.
This forms the basis of the pharmaceutical drugs industry. What medicines extracted from plants
and animals are used in your school or local dispensary?
7.Injection drugs and vaccines are made from plant or animal extracts
Apart from clothing, it is a tradition to put on shoes and other attire. Rubber shoes are made from
rubber. Rubber is a sticky milky fluid obtained from certain tropical trees. Skin shoes and
handbags are made from skins and hides of animals. The process of converting these raw
materials into the items mentioned above involves chemistry knowledge.
What other items made by chemical processes do you know?
Skin shoes
Sustainable crop and animal production is also enhanced by application of chemistry knowledge.
The use of chemicals in agriculture is inevitable. Fertilizers, insecticides, acaricides, herbicides
(weed killers) have and are still playing a good role in agricultural and animal production. In
some ecological zones, in order to get good harvest, fertilizer, herbicide and insecticide
application is necessary. The same case applies to animal production. As regards to control and
prevention of tick-borne diseases, application of different acaricides is often stressed. Also is the
use of different drugs to treat internal parasites such as worms, and vaccines to prevent certain
diseases.
8.The Importance of Chemistry in Daily Life
State the importance of Chemistry in daily life
There are a number of reasons for studying chemistry. If you ask someone to tell you the reason
for studying chemistry, he/she will give reasons based on how the subject touches him/her.
However, there are general and universal reasons as to why we should devote our valuable time
and energy to the study of chemistry. In general, we study chemistry because it helps as to
understand:
the composition of materials around us;
the nature, properties and behaviour of these materials;
why and how materials behave as they do;
how a new material, based on the known properties of its allies or counterparts might
behave;
how to make new materials which will be useful to us; and
how to extract and use materials from the earth to improve our welfare.
In economic and occupational terms, we can say that the knowledge of chemistry helps us:
to produce professionals in different disciplines such as pharmacy, engineering, medical
and natural science professions; and
make items, goods and materials for sale such as chemical laboratory equipments and
reagents, medicines, rubber, cement, paints, steel, plastics, etc. What other materials do you think
can be included in the list?
Therefore, we can summarize that the study of chemistry is important for survival, development
and welfare of man as well as sustainable production of crops and animals.
LABORATORY TECHNIQUES AND SAFETY
A laboratory is a room or building specially designed for conducting various scientific
experiments. An appropriate school laboratory has the following features:
a room with enough space for carrying out scientific experiments;
a store for keeping laboratory apparatus, chemicals and reagents;
an office for laboratory technician to sit in and design scientific experiments;
enough ventilation to let in fresh air and light;
wide doors and several exits for emergency evacuation in case of an accident; and
a wide table in front of the laboratory room, fitted with sinks for experiment
demonstrations by the teacher or technician.
Rules and safety precautions in a chemistry laboratory
laboratory Rules
State laboratory rules
Chemistry is best studied through doing experiments. Most experiments are conducted in the
laboratory. It is important to read and follow laboratory rules to avoid causing accidents. Your
teacher will teach and give you more rules. The following are some important laboratory rules:
1. Do not enter the laboratory without permission from your teacher or laboratory
technician.
2. Wear safety goggles all the time while in the laboratory. Obey this rule whether you are
actually working on an experiment or simply writing in your laboratory notebook.
3. Contact lenses are not allowed. Even when worn under safety goggles, various fumes
may accumulate under the lens and cause serious injuries or blindness.
4. Put on closed shoes and trousers when in the laboratory. Sandals and shots are strictly
prohibited.
5. Never walk or run unnecessarily in the laboratory.
6. Tie back long hair when using open flames.
7. Eating, drinking, and smoking are strictly prohibited in the laboratory.
8. Don‟t perform any experiment not authorized by your teacher or lab technician. If you
are curious about trying a procedure not covered in the experimental procedure, consult your
teacher or laboratory technician.
9. Never taste anything. Never directly smell the source of any vapour or gas; instead drift a
small sample to your nose. Do not inhale this vapour directly but take in only enough to detect an
odour if one exists.
10. Always wash your hands after experiments.
11. Never use your hands to transfer chemicals. Use a spatula instead.
12. Notify your teacher or technician immediately in case of an accident
13. Know what chemicals you are using, carefully read the label twice before taking anything
from the reagent bottle. Do not interchange labels.
14. Excess reagents are never to be returned to stock bottles. If you take too much, dispose of
the excess.
15. Many common reagents, for example, alcohol, acetone and carbon disulphide are highly
flammable. Do not use them anywhere near open flames.
16. Pour more concentrated solutions into less concentrated solutions to avoid violent
reactions. For example, always add acid to water; not water to acid. If you pour water into acid
instead, the heat of reaction will cause the water to explode into steam, sometimes violently, and
the acid will splash.
17. If chemicals accidentally splash onto your skin or eyes, flush immediately with plentiful
amounts of water and report to your teacher or lab technician.
18. Never point a test tube or vessel that you are heating at yourself or your colleague.
19. Dispose of chemicals properly. Unless you are told otherwise, assume that only water
may be poured in the laboratory sinks.
20. When an experiment is completed, always clean up your work area and dispose of the
broken glass properly. Return all equipment to its proper storage places.
21. Never take away anything from the laboratory without your teacher‟s permission.
22. Beware of hot glass because it looks exactly the same as a cold glass. Never touch it with
your hand.
23. Always adjust the Bunsen burner to give a luminous flame when not using it (or just
simply turn it off).
24. Use equipment or apparatus only for its designated use.
25. Never eat or drink from laboratory glassware.
26. Make sure all the burners are turned off before leaving the laboratory. Check that the gasNOTE: All the above rules and safety measures are applicable to all research, teaching and
academic laboratories. However, your laboratory may require some more rules that apply to
specific materials and equipment.
First aid and first aid kit
FIRST AID
15
First aid is the help given to someone who is injured or sick before the victim gets further
medical assistance. This help can be given by any person regardless of his/her knowledge in a
medical profession.
Whenever an accident occurs, something must be done immediately to help and save life of the
victim. You must always be ready to give a hand to a victim whenever an accident occurs close
to you. To give aid effectively and successfully, one must have elementary knowledge on how to
assist different victims. If you do not know how to help a certain victim, you can ask someone to
assist instead. Do not engage yourself in assisting if you actually do not know where to start.
You may find yourself worsening the situation of the victim unknowingly. However, this should
not be taken as an excuse for failing to help. Always be ready to render some kind of help. First
aid helps to:
1. relieve pain and bring hope to the victim.
2. prevent permanent disability
3. prevent the victim‟s condition from getting worse
4. reduce the possibility of death.
5. shorten recovery time
Possible Causes of Accidents in a Chemistry Laboratory
Identify possible causes of accidents in a chemistry laboratory
Accidents may occur in a school laboratory if utmost care is not taken into account. Accidents in
the laboratory are mainly cuts on parts of the body such as hands, fingers, legs or head. Others
are burns from flames, scalds from boiling fluids, bruises and grazes due to accidental falling on
a slippery floor.
Some possible causes of accidents in the laboratory include:
Failure to follow the correct experimental procedures for example, pouring water into an
acid instead of pouring an acid into water as the rule is.
Neglecting some laboratories rules such as ignoring to wear protective gears, tasting the
chemicals, eating or drinking while in the laboratory, etc.
16
Failure to adhere to proper conduct in the laboratory like running unnecessarily and
conducting experiments without your teacher's or technician's permission and guidance.
Improper use or handling of laboratory equipment and apparatus when conducting
experiments, which could lead to breakage and in turn cause cuts, bruises, grazes, etc.
A slippery laboratory floor which can cause fractures, cuts, bruises, grazes, etc
Accidental spillage of chemicals on body parts such as hands, face, eyes, etc, could lead
to burns and damage.
Poor ventilation in the laboratory may cause suffocation (due to inadequate oxygen
supply) and poisoning (by inhaling poisonous gases produced when experimenting).
Improper disposal of chemical wastes may result in explosions, burns or even fires.
The leaking of gases from taps or cylinders may cause fires or even explosions.
Use of wrong reagents due to incorrect labeling of chemicals or use of reagents or
chemicals that have expired may cause burns, poisoning or damage to apparatus or equipment.
Inadequate prior information or knowledge on procedures and hazards associated with
certain practical activities or reactants may result in burns, poisoning or explosions.
Loose or improperly plugged electrical appliances may cause electric shock, especially
when touched with wet hands and during fixing of sockets.
In general, it can be concluded that most laboratory accidents are a result of negligence and
carelessness of experimenters. It is also due to failure to follow the laboratory rules and general
safety measures.
The Items Found in a First Aid Kit
Name the items found in a first aid kit
A First Aid Kit is a box in which first aid chemicals, tools and instruments are kept. In the
laboratory, the box is usually kept in a place where it can be easily reached in case of an
accident, preferably on the wall.
17
Each student must be familiar with the tools and chemicals kept in the kit and learn how to use
them to provide first aid to a victim.
First aid kit and its contents
How Each First Aid Kit Item is Used
Demonstrate how each first aid kit item is used
The table below shows types of chemicals found in a First Aid Kit and their functions.
Tool/chemical/item Function
First aid manual Contains guidelines on how to use the items in the first aid kit
Sterile gloves
Worn on hands when attending bleeding cuts or wounds to
avoid infecting wounds and to prevent direct contact with the
victim‟s body fluids
Sterile dressing Stops bleeding
Antiseptic agent
Cleaning and disinfection of wounds, cuts, bruises, grazes or
blisters
Soap Washing hands, wounds and equipment
Antibiotic ointment Prevents infection on cuts and bruises in or near the eye
18
Burn ointment Applied on burns to prevent infection
Petroleum jelly Soothing broken skin
Plaster or adhesive bandage Covering small wounds or cuts
Sterile gauze Covering wounds to protect them from dirt or germs
Eye wash solution Flushing the eyes or as a general decontaminant
Thermometer Recording body temperature
Antibiotic towelettes or cotton wool Cleaning and drying cuts and wounds
Iodine tincture Dressing fresh cuts and bruises
Pain relieving drugs such as aspirin, paracetamol, panadol, etc Relieving mild pains
Liniment Reducing muscle pain
Mild antibiotics
Treating mild bacterial infections on the skin, ear, nose and
mouth
Gentian violet solution
Applied on minor wounds and treatment of serious heat
wounds
Hydrogen peroxide solution Cleaning wounds
Methylated spirit (70% alcohol) Cleaning cuts and bruises
Bandages Dressing wounds and cuts, and immobilizing injured limbs
Scissors or razor blade Cutting dressing materials
Dental kit Treatment of broken teeth, loss of crown or filling
Safety pins (small and big) Splinter removal and securing triangular bandage slings
19
Tweezers Splinter or stinger removal
Resealable oven bag Container for contaminated articles







academic laboratories. However, your laboratory may require some more rules that apply to
specific materials and equipment.
First aid and first aid kit
FIRST AID
15
First aid is the help given to someone who is injured or sick before the victim gets further
medical assistance. This help can be given by any person regardless of his/her knowledge in a
medical profession.
Whenever an accident occurs, something must be done immediately to help and save life of the
victim. You must always be ready to give a hand to a victim whenever an accident occurs close
to you. To give aid effectively and successfully, one must have elementary knowledge on how to
assist different victims. If you do not know how to help a certain victim, you can ask someone to
assist instead. Do not engage yourself in assisting if you actually do not know where to start.
You may find yourself worsening the situation of the victim unknowingly. However, this should
not be taken as an excuse for failing to help. Always be ready to render some kind of help. First
aid helps to:
1. relieve pain and bring hope to the victim.
2. prevent permanent disability
3. prevent the victim‟s condition from getting worse
4. reduce the possibility of death.
5. shorten recovery time
Possible Causes of Accidents in a Chemistry Laboratory
Identify possible causes of accidents in a chemistry laboratory
Accidents may occur in a school laboratory if utmost care is not taken into account. Accidents in
the laboratory are mainly cuts on parts of the body such as hands, fingers, legs or head. Others
are burns from flames, scalds from boiling fluids, bruises and grazes due to accidental falling on
a slippery floor.
Some possible causes of accidents in the laboratory include:
Failure to follow the correct experimental procedures for example, pouring water into an
acid instead of pouring an acid into water as the rule is.
Neglecting some laboratories rules such as ignoring to wear protective gears, tasting the
chemicals, eating or drinking while in the laboratory, etc.
16
Failure to adhere to proper conduct in the laboratory like running unnecessarily and
conducting experiments without your teacher's or technician's permission and guidance.
Improper use or handling of laboratory equipment and apparatus when conducting
experiments, which could lead to breakage and in turn cause cuts, bruises, grazes, etc.
A slippery laboratory floor which can cause fractures, cuts, bruises, grazes, etc
Accidental spillage of chemicals on body parts such as hands, face, eyes, etc, could lead
to burns and damage.
Poor ventilation in the laboratory may cause suffocation (due to inadequate oxygen
supply) and poisoning (by inhaling poisonous gases produced when experimenting).
Improper disposal of chemical wastes may result in explosions, burns or even fires.
The leaking of gases from taps or cylinders may cause fires or even explosions.
Use of wrong reagents due to incorrect labeling of chemicals or use of reagents or
chemicals that have expired may cause burns, poisoning or damage to apparatus or equipment.
Inadequate prior information or knowledge on procedures and hazards associated with
certain practical activities or reactants may result in burns, poisoning or explosions.
Loose or improperly plugged electrical appliances may cause electric shock, especially
when touched with wet hands and during fixing of sockets.
In general, it can be concluded that most laboratory accidents are a result of negligence and
carelessness of experimenters. It is also due to failure to follow the laboratory rules and general
safety measures.
The Items Found in a First Aid Kit
Name the items found in a first aid kit
A First Aid Kit is a box in which first aid chemicals, tools and instruments are kept. In the
laboratory, the box is usually kept in a place where it can be easily reached in case of an
accident, preferably on the wall.
17
Each student must be familiar with the tools and chemicals kept in the kit and learn how to use
them to provide first aid to a victim.
First aid kit and its contents
How Each First Aid Kit Item is Used
Demonstrate how each first aid kit item is used
The table below shows types of chemicals found in a First Aid Kit and their functions.
Tool/chemical/item Function
First aid manual Contains guidelines on how to use the items in the first aid kit
Sterile gloves
Worn on hands when attending bleeding cuts or wounds to
avoid infecting wounds and to prevent direct contact with the
victim‟s body fluids
Sterile dressing Stops bleeding
Antiseptic agent
Cleaning and disinfection of wounds, cuts, bruises, grazes or
blisters
Soap Washing hands, wounds and equipment
Antibiotic ointment Prevents infection on cuts and bruises in or near the eye
18
Burn ointment Applied on burns to prevent infection
Petroleum jelly Soothing broken skin
Plaster or adhesive bandage Covering small wounds or cuts
Sterile gauze Covering wounds to protect them from dirt or germs
Eye wash solution Flushing the eyes or as a general decontaminant
Thermometer Recording body temperature
Antibiotic towelettes or cotton wool Cleaning and drying cuts and wounds
Iodine tincture Dressing fresh cuts and bruises
Pain relieving drugs such as aspirin, paracetamol, panadol, etc Relieving mild pains
Liniment Reducing muscle pain
Mild antibiotics
Treating mild bacterial infections on the skin, ear, nose and
mouth
Gentian violet solution
Applied on minor wounds and treatment of serious heat
wounds
Hydrogen peroxide solution Cleaning wounds
Methylated spirit (70% alcohol) Cleaning cuts and bruises
Bandages Dressing wounds and cuts, and immobilizing injured limbs
Scissors or razor blade Cutting dressing materials
Dental kit Treatment of broken teeth, loss of crown or filling
Safety pins (small and big) Splinter removal and securing triangular bandage slings
19
Tweezers Splinter or stinger removal
Resealable oven bag Container for contaminated articles
Main Difference – Phase of Matter vs State of Matter
Matter is any substance that exists in the universe. Matter has a mass and a volume that occupy the space. Matter can exist in different forms according to the internal and external factors of matter. The same substance can exist in different forms at different temperatures, pressures, etc. The terms phase and state are used to describe these different forms of matter. Both these terms explain the same property of matter, but are different from each other according to several factors as discussed in this article. The main difference between phase of matter and state of matter is that phase of matter explains uniform chemical and physical properties of matter whereas state of matter explains the form of matter at a given temperature and a pressure.
Key Areas Covered
1. What is Phase of Matter
– Definition, Examples
2. What is State of Matter
– Definition, Examples3. What are the Similarities Between Phase of Matter and State of Matter
– Outline of Common Features4. What is the Difference Between Phase of Matter and State of Matter
– Comparison of Key Differences
– Definition, Examples
2. What is State of Matter
– Definition, Examples3. What are the Similarities Between Phase of Matter and State of Matter
– Outline of Common Features4. What is the Difference Between Phase of Matter and State of Matter
– Comparison of Key Differences
Key Terms: Gases, Liquid, Matter, Phase Of Matter, Pressure, Solid, State Of Matter, Temperature

What is Phase of Matter
Phase of matter is the form of matter that has uniform chemical and physical properties. In other words, phase of matter is a region of a substance that has uniform chemical and physical properties inside the boundary.
There are four major phases of matter. They are solid phase, liquid phase, gaseous phase and plasma phase. In these phases of matter, the density, index of refraction, chemical composition, etc. are uniform physical and chemical properties. The difference between these phases mainly depends on the arrangement of particles (these particles can be molecules, atoms or ions).
For a certain substance, the phase can be changed from one phase to a different phase. For example, by changing the temperature of water, liquid water phase can be converted into vapor phase (gaseous phase) of water. This is known as the phase transition.

Figure 1: Phase Diagram for Water
The phase transition can be explained by terms such as melting, freezing, vaporization, sublimation, condensation, etc. The above image shows the phase diagram of water. The letters S, L and V stand for solid phase, liquid phase, and vapor phase respectively. TP indicates the triple point of water. It gives the temperature and pressure in which water can exist in all three phases at the same time as an equilibrium.
What is State of Matter
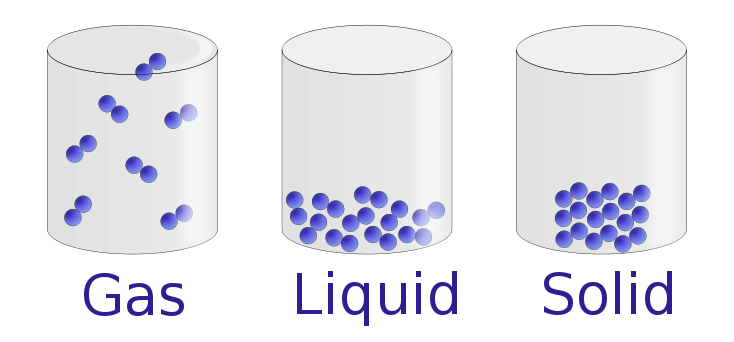
State of matter is the form of matter at a given temperature and pressure. The state of matter depends on the temperature and pressure. At a given temperature and pressure a substance can exist mainly in one of four major states of matter: solid state, liquid state, gaseous state and plasma state of matter.

Figure 2: The Three Main States of Matter
It is not essential to have the uniform physical and chemical properties in a substance when considering the state of mater. Therefore, there can be several phases in one state of matter. This happens when these phases are immiscible with each other but are in the same state of matter.
The state of matter can be changed by changing the temperature of a substance since the change in temperature can change the internal energy of a substance. Hence, the kinetic energy of particles in that substance is changed. It causes the state of matter to be changed.
Similarities Between Phase of Matter and State of Matter
- Both terms explain the form of matter.
- Both types include the same forms of matter: solid, liquid. Gas and plasma.
Difference Between Phase of Matter and State of Matter
Definition
Phase of Matter: Phase of matter is the form of matter that has uniform chemical and physical properties.
State of Matter: State of matter is the form of matter at a given temperature and pressure.
Nature of Properties
Phase of Matter: A phase of matter has uniform properties.
State of Matter: A state of matter may or may not have uniform properties.
Phase
Phase of Matter: A phase of matter has only one phase.
State of Matter: A state of matter can have several phases.
Transition
Phase of Matter: The change in phase does not change the uniformity of matter.
State of Matter: The change in state may or may not change the uniformity of matter.
Conclusion
Both phase of matter and state of matter explain the same concept, i.e., the form of matter. But they are two separate terms when the definitions are considered. The main difference between phase of matter and state of matter can be given as: phase of matter explains uniform chemical and physical properties of matter whereas state of matter explains the form of matter at a given temperature and a pressure.
Main Difference – Element vs Molecule vs Compound
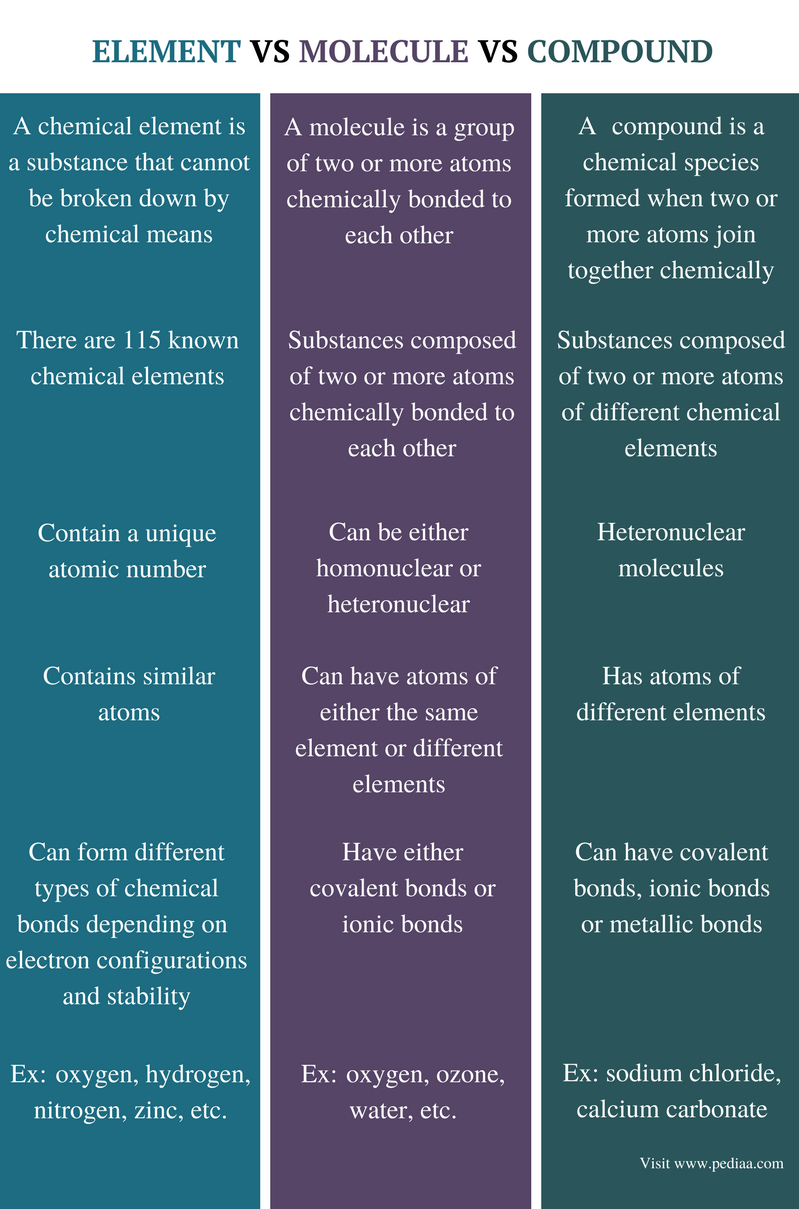
The terms element, molecule, and compound, have different definitions and properties as discussed below in this article. Although often we use the term compound to name any molecule, not all compounds are only molecules. There are many other chemical species that we can call a compound. An element is a substance that cannot be broken down further by chemical means. A molecule is a substance that is made out of two or more atoms bonded via chemical bonds. A compound is a molecule composed of different types of atoms bonded via chemical bonds. Therefore, a compound is also a type of molecule, but they are not the same. The main difference between element, molecule and compound is that an element is a substance that cannot be further divided into parts by chemical means whereas a molecule is a substance that can be further divided into parts by chemical means and a compound is also a type of molecule but is composed of different types of molecules.
Key Areas Covered
1. What is an Element
– Definition, Periodic Table, Reactivity, Isotopes
2. What is a Molecule
– Definition, Chemical Formula, Different Types
3. What is a Compound
– Definition, Different Types
4. What is the Relationship Between Element Molecule and Compound
5. What is the Difference Between Element Molecule and Compound
– Comparison of Key Differences
– Definition, Periodic Table, Reactivity, Isotopes
2. What is a Molecule
– Definition, Chemical Formula, Different Types
3. What is a Compound
– Definition, Different Types
4. What is the Relationship Between Element Molecule and Compound
5. What is the Difference Between Element Molecule and Compound
– Comparison of Key Differences
Key Terms: Atom, Atomic Number, Element, Chemical Bond, Compound, Covalent Bond, Electron Configuration, Ionic Bond, Mass Number, Molecule

What is an Element
A chemical element is a substance that cannot be broken down by chemical means. Many different chemical elements have been discovered so far. They have a unique property, i.e., the number of protons in the nucleus. This is called the atomic number. The atomic number of an element is a fixed value for a certain element. Two elements cannot have the same atomic number. Any change in the atomic number changes the element. However, elements can be changed through nuclear reactions.
Periodic Table
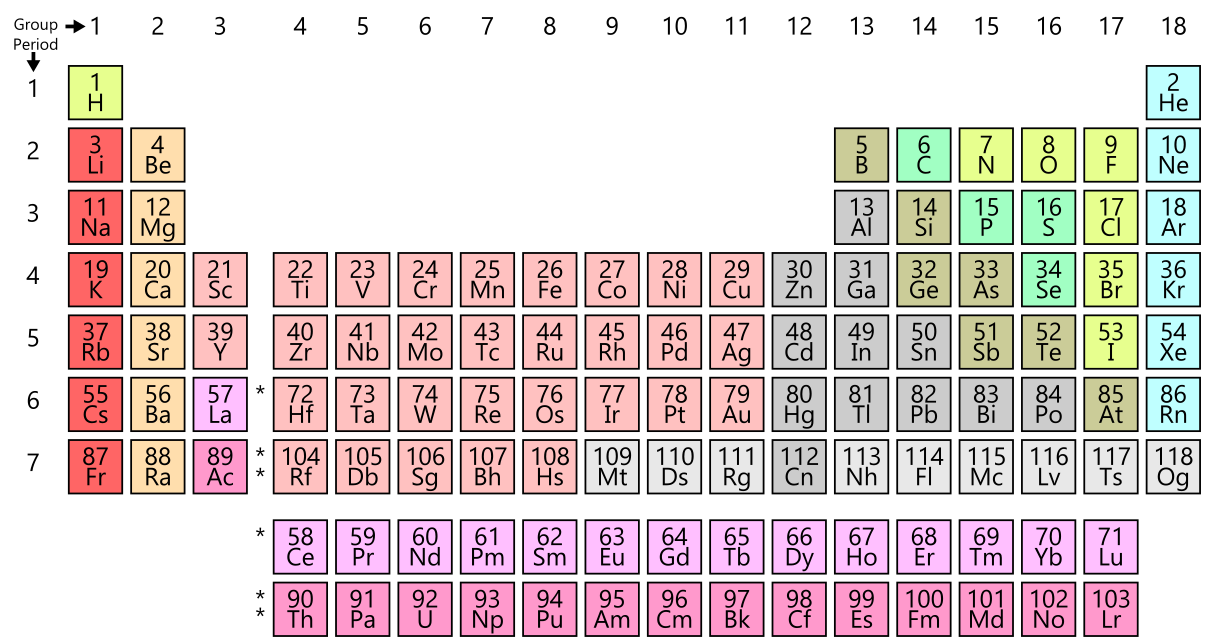
Chemical elements are arranged in the periodic table of elements based on their atomic number and the electron configuration. A chemical element can also be explained as a species of atoms or a group of atoms. This is because the atoms that can be found anywhere belong to a certain chemical element. This happens because of the uniqueness of the atomic number for a certain chemical element.

Figure 1: Periodic Table of Elements
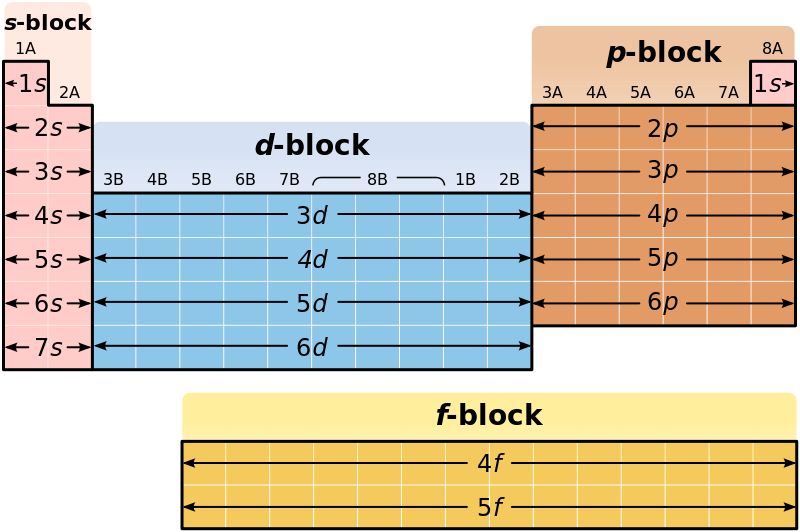
In the periodic table of elements, there are different categories of chemical elements. Some of the classifications are shown below.
- Metals, non-metals, and metalloids
- s block elements, p block elements, d block elements and f block elements.
- Alkali metals, alkaline earth metals, transition metals.
- Halogens, noble gases, etc.
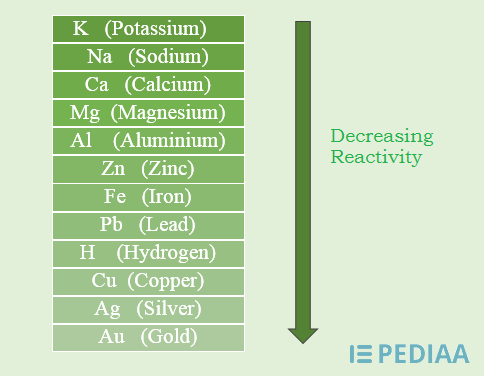
Reactivity
Some chemical elements are inert; some are less reactive while some are very reactive. Inert chemical elements include the group of noble gasses. All other elements can undergo chemical reactions easily. This is because, they do not have incomplete electron shells according to the electron configuration of noble gases, and thus are very stable as individual atoms. They have no reason to react with other elements. But the other chemical elements have incomplete electron configurations. Therefore, they undergo different chemical reactions in order to fill their electron shells. The less reactive chemical elements have a partially filled, yet stable electron configurations.

Figure 2: Metal Activity Series
Isotopes
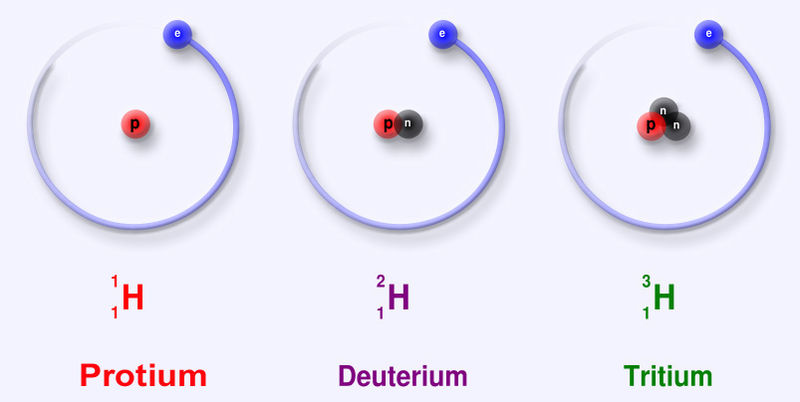
Some chemical elements are highly radioactive since they are highly unstable. They decay over time until they get a stable state. Some chemical elements have different forms known as isotopes. Isotopes of a certain chemical element have the same atomic number but a different mass number. This means, the number of protons in their nuclei is the same; hence, they belong to the same chemical element. But the number of neutrons in the nuclei are different from each other.

Figure 3: Isotopes of Hydrogen Chemical Element
There are names and symbols used to name each and every element. Most of these names are Latin words, and the symbols are derived accordingly.
What is a Molecule
A molecule is a group of two or more atoms chemically bonded to each other and is neutral. A molecule may contain the atoms of the same chemical element or different chemical elements. A molecule in chemistry is a polyatomic chemical species with a neutral electrical charge. Molecules can be categorized into different groups depending on the chemical and physical properties of molecules.
A molecule may contain atoms bonded via either covalent bonds or ionic bonds. A covalent bond is formed when two atoms share their unpaired electrons. An ionic bond is an electrostatic attraction between two or more atoms.
Chemical Formula
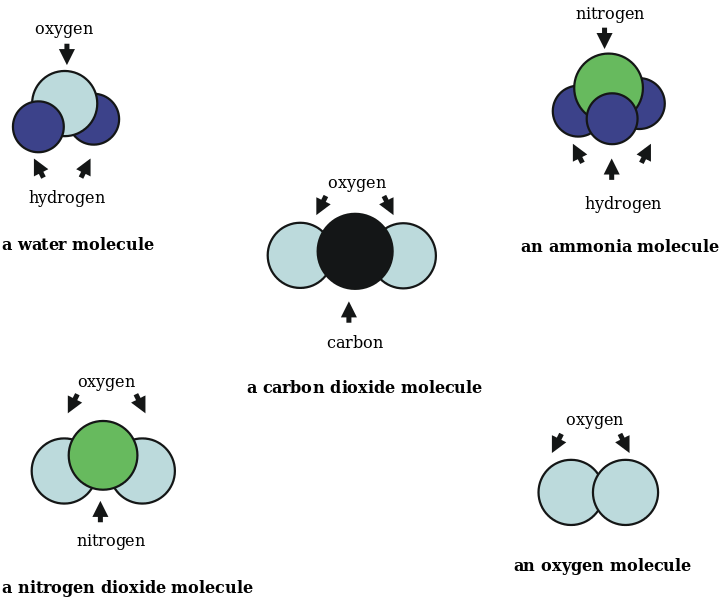
The composition of atoms in a molecule is given by its chemical formula. The empirical formula gives the ratio between the atoms. For example, C3H6 is the chemical formula of propene. There are three carbon atoms and six hydrogen atoms bonded to each other. The empirical formula for this molecule is CH2.

Figure 4: Some Common Molecules
Different Types of Molecules
- Based on the types of atoms present in a molecule, it can be either homonuclear or heteronuclear. Homonuclear molecules are composed of atoms of the same element. Heteronuclear molecules are composed of atoms of different chemical elements.
- Molecules can be either organic or inorganic. Organic molecules are composed of essentially C,H along with some other elements. Inorganic molecules may have different combinations of different chemical elements.
- Number of atoms per molecule: diatomic molecules, triatomic molecules, polyatomic molecules.
- Molecules composed only of covalent bonding are covalent molecule, and the molecules containing ionic bonding are ionic molecules.
- According to geometry, molecules can be either symmetrical or asymmetrical molecule. For example, the linear geometry of CO2 makes it a symmetrical molecule.
Likewise, there are many types of molecules that can be found in nature. They have different abundances. The individual atoms are not molecules. For example, Helium (He) is not a molecule.
What is a Compound
A compound is a chemical species that is formed when two or more atoms join together chemically, with covalent or ionic bonds. All compounds are molecules, but not all molecules are compounds. Homonuclear molecules are not compounds. Only heteronuclear molecules are considered as compounds. Compounds can be grouped in different ways, only a few are mentioned below.
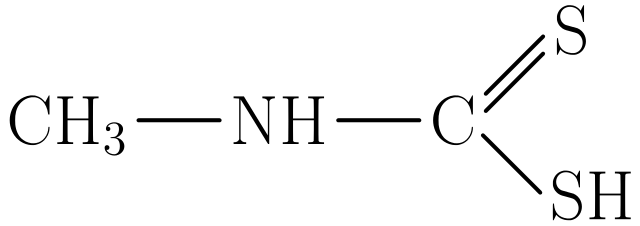
Figure 5: This is a covalent compound that contains atoms of different chemical elements
Different Types of Compound
- Based on the number of atoms, compounds can be diatomic, triatomic or polyatomic.
- Based on the type of chemical bonding, covalent compounds contain covalent bonds, and ionic compounds contain ionic bonds.
- Based on the complexity, some compounds are simple compounds while other are complex compounds.
- Based on the components (cation, anions, ), compounds can be organic compound or inorganic compound. Organic compounds include hydrocarbons, carboxylic acids, amides, ammines, alcohols, etc. Inorganic compounds include oxides, hydrides, halides, nitrites, nitrates, carbonates, etc.
Relationship Between Element Molecule and Compound
- Molecules are made out of atoms of same or different chemical elements. Molecules containing different types of elements are called compounds.
Difference Between Element Molecule and Compound
Definition
Element: A chemical element is a substance that cannot be broken down by chemical means.
Molecule: A molecule is a group of two or more atoms chemically bonded to each other.
Compound: A compound is a chemical species that is formed when two or more atoms join together chemically, with covalent or ionic bonds.
Members
Element: There are 115 known chemical elements.
Molecule: Substances composed of two or more atoms chemically bonded to each other are molecules.
Compound: Substances composed of two or more atoms of different chemical elements are compounds.
Unique Properties
Element: Chemical elements contain a unique atomic number.
Molecule: Molecules can be either homonuclear or heteronuclear.
Compound: Heteronuclear molecules are compounds.
Chemical Element
Element: An element contains similar atoms.
Molecule: A molecule can have atoms of either the same element or different elements.
Compound: A compound has atoms of different elements.
Chemical Bonding
Element: Atoms of different elements can form different types of chemical bonds depending on their electron configurations and the stability.
Molecule: Molecules can have either covalent bonds or ionic bonds.
Compound: Compounds can have covalent bonds, ionic bonds or metallic bonds.
Examples
Element: Some examples for chemical elements include oxygen, hydrogen, nitrogen, copper, zinc, etc.
Molecule: Some examples for molecules include oxygen (O2), ozone (O3), water (H2O), etc.
Compound: Some examples for compounds include sodium chloride (NaCl), calcium carbonate (CaCO3), etc.
Conclusion
Molecules are composed of chemical elements. Molecules that contain two or more different chemical elements are known as compounds. The main difference between element molecule and compound is that an element is a substance that cannot be further divided into parts by chemical means whereas a molecule is a substance that can be further divided into parts by chemical means and a compound is also a type of molecule but is composed of different types of molecules.
Main Difference – d vs f Block Elements
A chemical element is any material that cannot be broken down or changed by chemical means. There are 118 known chemical elements. These chemical elements are the building blocks of matter. All chemical elements are arranged in the periodic table of elements, in the order of increasing atomic number. There are also four groups of elements in the periodic table: s block, p block, d block and f block. Elements are grouped into these groups based on their electron configurations. For example, s block elements have their outermost electrons in an s orbital. p block elements have their outermost electrons in a p orbital. The main difference between d block elements and f block elements is that d block elements are chemical elements having electrons filled to their d orbitals whereas f block elements are chemical elements having electrons filled to their f orbitals.
Key Areas Covered
1. What are d Block Elements
– Definition, Chemical Properties
2. What are f Block Elements
– Definition, Chemical Properties, Lanthanides and Actinides
3. What is the Difference Between d and f block Elements
– Comparison of Key Differences
– Definition, Chemical Properties
2. What are f Block Elements
– Definition, Chemical Properties, Lanthanides and Actinides
3. What is the Difference Between d and f block Elements
– Comparison of Key Differences
Key Terms: Actinides, Aufbau Principle, d Block, Electron Configuration, f Block, Inner Transition Elements, Lanthanides, Orbitals, Periodic Table

What are d Block Elements
d block elements are chemical elements having electrons filled to their d orbitals. The very first requirement for an element to be a d block element is the presence of d orbitals. Elements having at least one electron in their d orbitals are categorized as d block elements. The d-block of the periodic table is located between the s-block and the p-block.
One important fact about d block elements is that they have d orbitals that are partially or completely filled with electrons. According to Aufbau principle, electrons fill orbitals according to the ascending order of the energies of orbitals. In other words, electrons fill the ns orbital before filling the (n-1) d orbital. This is because the energy of ns orbital is lower than (n-1) d orbital. In elements of the first row of the periodic table, electrons first fill the 4s orbital before filling the 3d orbital.

Figure 1: Four Major Groups of the Periodic Table
But there are some exceptions as well. Although the energy level is lower, sometimes electrons fill the orbitals with the most stable electron configuration. For example, ns1nd10 configuration is more stable than ns2nd9. That is due to the stability of the complete filling of the d orbitals. Such two examples are shown below.
Chromium (Cr) = [Ar]3d54s1
Copper (Cu) = [Ar]3d104s1
All d block elements are metals. They show very high melting points and boiling points due to their strong metallic bonds. The decreasing of the atomic radii is slight compared to that of s and p block elements. Moreover, the densities are very high due to the metallic nature. Due to the presence of d electrons, d block elements show variable oxidation states.
What are f Block Elements
f block Elements are chemical elements having electrons filled to their f orbitals. The f block is shown in the periodic table as a separate group at the bottom of the periodic table. That is because they have electrons filling up the f orbitals that are shielded by other orbitals; hence, f block elements are known as “inner transition elements”. The true position of the f block in the periodic table is between the s block and d block. These elements are known as rare elements because most of these elements are rarely found on earth.
There are two series of the f block elements named as,
- Lanthanide series (elements are known as Lanthanides)
- Actinide series (elements are known as Actinides)
These two series are named as such according to the element from which the series starts with. Lanthanide series starts immediately after Lanthanum (La) and actinide series starts with Actinium (Ac). All Lanthanides and Actinides are metals.

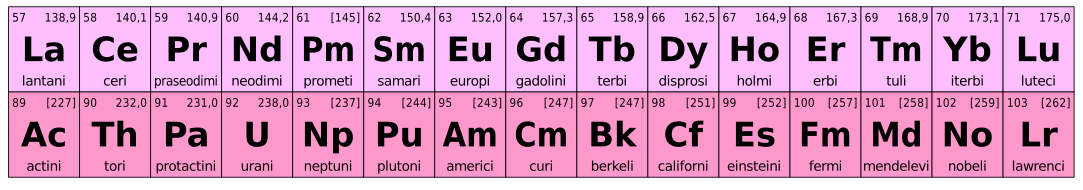
Figure 2: Lanthanides and Actinides
Lanthanide Series
Lanthanide series contains 14 elements that start immediately after Lanthanum. Therefore, this series contains a total of 15 elements along with Lanthanum. The atomic number of the series is from 57 to 71. They are known as “first inner transition series”. Lanthanides belong to the 4f series since these elements have their electrons filling to the 4f orbitals. But, Lanthanum has a completely empty f subshell; thus, the elements from Cerium (Ce) to Lutetium (Lu) are considered as the lanthanides.
The 4f electrons of these elements are completely shielded by other orbitals and do not take part in any chemical bonding. The Lanthanides are silvery-white metals and are good conductors of heat. The elements having completely or half-filled f orbitals are stable than other elements of the series.
The most stable oxidation state Lanthanides show is +3. Some elements show +2 and +4 oxidation states as well, but they are not stable as +3 oxidation state. Lanthanides are highly reactive and can react with elements such as hydrogen, oxygen, carbon, etc.
Almost all the ions formed by lanthanides are colourless. Lanthanides are electropositive elements. Therefore, they prefer to form molecules with electronegative elements. However, throughout the series, the changes of the chemical and physical properties are very less.
Actinide Series
Actinides are chemical elements that can be found in the actinide series of the f block in the periodic table of elements. All actinides are radioactive elements due to their unstable nature. These elements are composed of very large atoms. Actinides have their valence electrons in the 5f orbital. The actinide series is composed of chemical elements having the atomic numbers 89 to 103.
The most common and abundant actinides on earth are Uranium and Thorium. They are weakly radioactive and release high energy during radioactive decay. The prominent oxidation state among actinides is +3. In addition, actinides show oxidation states such as +4, +5 and +6.
Actinides form basic oxides and hydroxides. They have the ability to form complexes with ligands such as chlorides, sulfates, etc. Most complexes of actinides are colourful. However, due to the radioactivity and heavy metal behaviour, actinides are considered as toxic compounds.
Difference Between d and f Block Elements
Definition
d Block Elements: d block elements are chemical elements having electrons filled to their d orbitals.
f Block Elements: f block elements are chemical elements having electrons filled to their f orbitals.
Other Names
d Block Elements: d block elements are known as “transition elements”.
f Block Elements: f block elements are known as “inner transition elements”.
Oxidation States
d Block Elements: d block elements show a wide range of oxidation states depending on their electron configurations.
f Block Elements: The most stable oxidation state for f block elements is +3, and there can be other oxidation states as well.
Stability
d Block Elements: Almost all the elements in the d block are stable.
f Block Elements: Most f block elements are radioactive.
Groups
d Block Elements: d block elements can be either transition elements or non-transition elements.
f Block Elements: f block elements are in two series as Lanthanides and Actinides.
Electron Configuration
d Block Elements: d block elements have partially or completely filled outermost d orbitals.
f Block Elements: f block elements are unified by having one or more of their outermost electrons in the f orbital.
Conclusion
The periodic table of elements shows the arrangement of all known chemical elements according to their atomic numbers. There are four major groups of chemical elements that have similar chemical and physical properties among the members of each group. The d block and f block are two groups among those four groups. The main difference between d block elements and f block elements is that d block elements are chemical elements having electrons filled to their d orbitals whereas f block elements are chemical elements having electrons filled to their f orbitals.
Main Difference – Burning vs Combustion
Combustion typically refers to the process of burning something. It is an exothermic reaction which releases heat and light as energy forms. Combustion reactions generally take place when a hydrocarbon or a fuel reacts with oxygen. In other words, hydrocarbons are oxidized by molecular oxygen. Combustion reactions can occur as either complete combustions or incomplete combustions. Both methods form byproducts such as carbon dioxide (CO2), carbon monoxide (CO) and water (H2O). Burning is also a type of combustion. The main difference between burning and combustion is that burning essentially cause the creation of a flame whereas combustion may or may not create a flame.
Key Areas Covered
1. What is Burning
– Definition, Properties, Examples
2. What is Combustion
– Definition, Properties, Complete and Incomplete Combustion, Examples
3. What are the Similarities Between Burning and Combustion
– Outline of Common Features
4. What is the Difference Between Burning and Combustion
– Comparison of Key Differences
– Definition, Properties, Examples
2. What is Combustion
– Definition, Properties, Complete and Incomplete Combustion, Examples
3. What are the Similarities Between Burning and Combustion
– Outline of Common Features
4. What is the Difference Between Burning and Combustion
– Comparison of Key Differences
Key Terms: Burning, Carbon Dioxide, Carbon Monoxide, Combustion, Complete Combustion, Flame, Incomplete Combustion

What is Burning
Burning refers to setting something on fire. In order to burn something, there should be three things: a flammable material, oxygen, and fire. The major characteristic of burning is the creation of a flame. When a flammable material is burnt, a flame appears. The color of the flame depends on the amount of oxygen present and the type of material that is going to be burnt.
Burning is a type of combustion since a material is oxidized by molecular oxygen and form byproducts such as carbon dioxide (CO2), carbon monoxide (CO), carbon dust or soot (C) and water (H2O). If the combustion reaction that occurs in burning is a complete oxidation, then the flame is blue colored. But if it is an incomplete combustion, the flame would be a yellow-orange colored one.

Figure 1: Burning of wood results in a yellow colored flame.
The heat energy produced by burning is comparatively less. This is because some of the energy is released as light energy due to the formation of a flame. Burning causes the formation of smoke. Burning of wood causes the formation of wood smoke. This smoke is composed of tiny particles which are unburnt. Smoke is a result of incomplete combustion.
What is Combustion
Combustion is a chemical reaction that involves the oxidation of a fuel. This is an exothermic reaction, which releases heat and light as energy forms. The combustion occurs in the presence of oxygen. Therefore, molecular oxygen act as the oxidizing agent.
Combustion may occur in two ways: complete combustion or incomplete combustion. Generally, complete combustion is characterized by a blue flame whereas incomplete combustion is characterized by yellow colored flame. However, combustion reactions do not always produce a flame. When a flame is not formed, that combustion yields a high amount of energy. This is because almost all the energy produced from the combustion is turned into heat, not light.

Figure 2: Petrol can undergo combustion without forming a flame. Therefore it is used in vehicle engines.
The complete combustion mainly results in carbon dioxide (CO2) and water (H2O). Incomplete combustion reactions result in carbon monoxide (CO) and water along with snoot. Both flammable substances and inflammable substances can undergo combustion. Inflammable substances such as petrol do not form a flame but are oxidized by oxygen.
Similarities Between Burning and Combustion
- Burning is also a type of combustion reaction.
- Both burning and combustion give byproducts such as CO2, CO, and H2
- Both reactions release heat energy.
- Both types involve oxidation of a material by oxygen.
Difference Between Burning and Combustion
Definition
Burning: Burning is setting something on fire.
Combustion: Combustion is a chemical reaction that involves the oxidation of a fuel.
Flame
Burning: Burning always creates a flame.
Combustion: Combustion may or may not create a flame.
Heat Energy
Burning: Burning forms a low amount of heat energy.
Combustion: Combustion forms a high amount of energy.
Light Energy
Burning: Burning always produce light energy.
Combustion: Combustion may or may not form light as an energy form.
Summary – Burning vs Combustion
Burning and combustion are often the same. The main difference between burning and combustion is the formation of a flame. Combustion reactions that form a flame can be grouped as burnings. However, both burning and combustion produce heat energy. The heat energy produced by combustion reactions are mainly used in industrial purposes. Heat energy produced from burning is mainly used to fulfill household needs, such as wood burning for cooking purposes.
Main Difference – Complete Combustion vs Incomplete Combustion
A combustion reaction is a chemical reaction that releases energy by the oxidation of a fuel. The chemical reactions which release energy are called exothermic reactions. Thus, combustion reactions are exothermic. A fuel can be oxidized with an oxidizing agent. The oxidizing agent for most combustion reactions is atmospheric oxygen. The energy that is released from combustion reactions can be either heat or light. Energy is mainly released in the form of heat; light energy is also released as a flame. Combustion can occur in two ways as complete combustion and incomplete combustion. The main difference between complete combustion and incomplete combustion is that in complete combustion, carbon dioxide is the only product that includes carbon whereas, in incomplete combustion, carbon monoxide and carbon dust are formed as products.
Key Areas Formed
1. What is Complete Combustion
– Definition, Properties, Examples
2. What is Incomplete Combustion
– Definition, Properties, Examples
3. What are the Similarities Between Complete Combustion and Incomplete Combustion
– Outline of Common Features
4. What is the Difference Between Complete Combustion and Incomplete Combustion
– Comparison of Key Differences
– Definition, Properties, Examples
2. What is Incomplete Combustion
– Definition, Properties, Examples
3. What are the Similarities Between Complete Combustion and Incomplete Combustion
– Outline of Common Features
4. What is the Difference Between Complete Combustion and Incomplete Combustion
– Comparison of Key Differences
Key Terms: Carbon Dioxide, Carbon Monoxide, Combustion, Exothermic Reaction, Flame, Fuel, Oxidation, Oxidizing Agent

What is Complete Combustion
Complete combustion is the complete oxidation of fuel. This reaction is highly exothermic and produces a high amount of energy and a limited number of products. In fuel burning or combustion, hydrocarbons in the fuel are oxidized by atmospheric oxygen, giving carbon dioxide (CO2) and water (H2O) as products. The complete combustion occurs where there is a sufficient amount of oxygen present. In the presence of oxygen, carbon atoms in hydrocarbons can get oxidized into carbon dioxide and hydrogen is oxidized into water. The general reaction for complete combustion is given below.
Hydrocarbon + Oxygen → Carbon dioxide + Water
For a fuel like ethanol, the complete combustion can be given as,
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
The complete combustion reactions result in oxides of carbon, sulfur and other elements present in the fuel. Carbon is oxidized to carbon dioxide whereas sulfur is oxidized into sulfur dioxide. Complete combustion results in less amount of air pollutants. The complete combustion can be generally characterized by a blue flame.

Figure 01: A blue flame is created in complete combustion.
Since the atmosphere is composed of only 21% oxygen by volume, a lot of air is needed for complete combustion to take place. Even though the amount of by products that result from complete combustion is low, it still adds unfavourable emissions. For example, carbon dioxide is a greenhouse gas which causes global warming.
What is Incomplete Combustion
Incomplete combustion is a chemical reaction that involves the partial oxidation of a fuel. The incomplete combustion occurs where there is an insufficient amount of oxygen. Here, the fuel is incompletely oxidized. Hence, the incomplete combustion forms a number of byproducts. But the amount of energy released from this combustion is comparatively low. The major products of incomplete combustion include Carbon monoxide (CO), carbon dust (we call it “soot”) and water (H2O). The general formula for incomplete combustion is shown below.
Hydrocarbon + Oxygen → Carbon monoxide + Carbon + Water
The byproducts may vary according to the amount of oxygen that is involved in the combustion. For example, sometimes it yields only carbon monoxide or soot. However, it commonly gives a mixture of carbon monoxide and soot along with water.
For example, incomplete combustion of ethylene can result in carbon and water as byproducts.
C2H4(l) + O2(g) → 2C(s) + 2H2O(g)
Incomplete combustion of ethanol can form carbon monoxide and carbon dust along with water.
C2H5OH(l) + 2O2(g) → 2CO(g) + 3H2O(l)
C2H5OH(l) + O2(g) → C(s) + 3H2O(l)

Figure 2: A yellow flame is produced with incomplete combustion.
Incomplete combustion is characterized by a yellow flame. Since the amount of energy released from incomplete combustion is low, it is undesirable. Moreover, carbon monoxide produced by this combustion is an air pollutant and is deadly for the human body. Carbon monoxide can bind with hemoglobin in our blood and limit the oxygen transportation inside the body.
Similarities Between Complete Combustion and Incomplete Combustion
- Both complete combustion and incomplete combustion are exothermic.
- They produce heat and light as energy forms.
- Both reactions give water as a byproduct.
- Both combustion types involve the oxidation of a fuel.
- These reactions involve the combination of molecular oxygen with fuel.
- Both these combustion reactions result in unfavorable gas emissions.
- They can form flames while burning.
Difference Between Complete Combustion and Incomplete Combustion
Definition
Complete Combustion: Complete combustion is the complete oxidation of fuel.
Incomplete Combustion: Incomplete combustion is the partial oxidation of fuel.
Energy Released
Complete Combustion: Complete combustion produces a high amount of energy.
Incomplete Combustion: Incomplete combustion produces a low amount of energy.
Amount of Oxygen Involved
Complete Combustion: Complete combustion occurs where there is enough oxygen present.
Incomplete Combustion: Incomplete combustion occurs where there isn’t enough oxygen present.
Byproducts
Complete Combustion: Complete Combustion produces carbon dioxide and water as major byproducts.
Incomplete Combustion: Incomplete combustion produces carbon monoxide, carbon dust and water as major byproducts.
Flame
Complete Combustion: Complete combustion creates a blue colored flame.
Incomplete Combustion: Incomplete combustion creates a yellow colored flame.
Effect on the Environment
Complete Combustion: Complete Combustion produces carbon dioxide which can cause global warming.
Incomplete Combustion: Incomplete combustion produces carbon monoxide which is an air pollutant.
Conclusion
Combustion reactions are exothermic reactions that release energy when fuel is burnt. Complete combustion of a fuel yields a high amount of energy whereas incomplete combustion yields a less amount of energy. This is the main difference between complete combustion and incomplete combustion. Complete combustion is very important in industrial scale applications. Incomplete combustion is used in household needs such as burning wood to make heat energy for cooking, etc. Even though there are a number of uses in combustion, it causes the emission of unfavorable gases to the environment that can act as air pollutants.
Main Difference – Combustion vs Pyrolysis
Combustion and pyrolysis are thermochemical reactions. Combustion is an exothermic chemical reaction; the combustion of a fuel can form light and heat as forms of energy. Pyrolysis is a decomposition reaction; here, organic materials are decomposed when provided with heat. Although both these processes are thermochemical reactions, there are differences between the two processes. The main difference between combustion and pyrolysis is that combustion is done under the presence of oxygen whereas pyrolysis is done under the absence (or near absence) of oxygen.
Key Areas Covered
1. What is Combustion
– Definition, Complete and Incomplete Combustion, Uses
2. What is Pyrolysis
– Definition, Process, and Uses
3. What is the Difference Between Combustion and Pyrolysis
– Comparison of Key Differences
Key Terms: Burning, Combustion, Complete Combustion, Decomposition, Exothermic Reaction, Incomplete Combustion, Pyrolysis, Thermochemical Reactions
– Definition, Complete and Incomplete Combustion, Uses
2. What is Pyrolysis
– Definition, Process, and Uses
3. What is the Difference Between Combustion and Pyrolysis
– Comparison of Key Differences

What is Combustion
Combustion is a chemical reaction that involves the reaction between substances and oxygen, producing light and heat as forms of energy. This process is also called burning. Heat and light are the energy forms given as the outcome of the reaction. Light energy appears as a flame. However, most of the energy is released as heat energy.
Combustion reaction can be found in two types as complete combustion and incomplete combustion.
Complete Combustion
Complete combustion occurs when an excess of oxygen is present for the reaction. This gives a limited number of products. When fuel is burnt, it gives carbon dioxide and water as the end products. When an element is burnt, it gives the most stable oxide of that element.
Incomplete Combustion
Incomplete combustion occurs in the presence of a low amount of oxygen. This gives more products at the end. When fuels undergo incomplete combustion, it gives carbon monoxide along with carbon dioxide and water. Sometimes, even unburnt carbon element is also produced. Here, carbon is released as soot.

Figure 1: Fire is a Result of Combustion
There are many uses of combustion reaction. One major use is the production of energy by burning fuels. This is important in industries, automobiles, etc. Producing fire is another use. Fire is used for many needs such as cooking. Sometimes, combustion of elements is used to identify elements. Different elements give different colored flames when combusted. By observing these flames, we can identify different elements.

Figure 1: Fire is a Result of Combustion
What is Pyrolysis
Pyrolysis is the chemical decomposition of organic materials in the absence of oxygen. This process requires the application of heat. Therefore, the rate of pyrolysis can be increased by increasing the temperature.
Pyrolysis typically occurs at temperatures above 430oC. However, since it is very difficult to obtain an oxygen-free atmosphere for pyrolysis, it is done under conditions with near absence of oxygen. Pyrolysis gives end products in gaseous phase, liquid phase, and solid phase. Most substances are converted into gases. Sometimes a little amount of liquid is formed. This liquid is called tar. The solid residue typically includes charcoal and biochar.
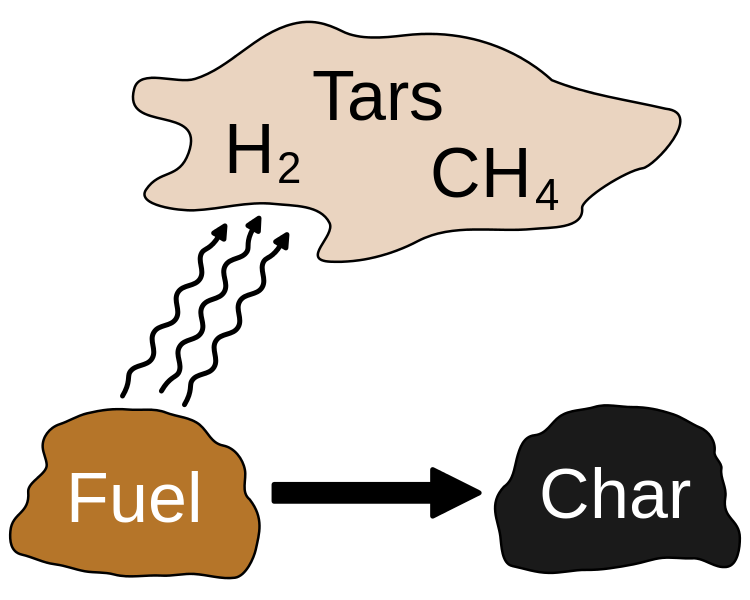
Figure 2: Products of Pyrolysis
The process of pyrolysis transforms organic material into their gaseous components, a solid residue of carbon and ash, and a liquid called pyrolytic oil. In pyrolysis, there are two methods that are used to remove any contaminants from a substance. They are destruction and removal. In destruction, contaminants are broken down into smaller compounds. But in the process of removal, rather than breaking compounds, contaminants are separated.
Pyrolysis is useful in industries for the production of charcoal, activated carbon, methanol, etc. Apart from that, pyrolysis treats and destroys semi-volatile organic compounds, fuels, and pesticides in soil. This process can also be used to treat organic waste coming out from factories.

Figure 2: Products of Pyrolysis
Difference Between Combustion and Pyrolysis
Definition
Combustion: Combustion is a chemical reaction that involves the reaction between substances and oxygen producing light and heat as forms of energy.
Pyrolysis: Pyrolysis is the chemical decomposition of organic materials in the absence of oxygen.
Atmosphere
Combustion: Combustion is done under the presence of oxygen in the atmosphere.
Pyrolysis: Pyrolysis is done under the absence of oxygen.
End Products
Combustion: Combustion produces gaseous end products.
Pyrolysis: Pyrolysis produces gaseous components along with trace amounts of liquid and solid residues.
Types
Combustion: Combustion may occur as complete combustion or incomplete combustion.
Pyrolysis: Pyrolysis can be done in a vacuum or in an atmosphere with near absence of oxygen.
Conclusion
Combustion and pyrolysis are thermochemical reactions. These reactions are used in industries and daily needs. These processes are different from each other in several factors. However, the main difference between combustion and pyrolysis is that combustion is done under the presence of oxygen whereas pyrolysis is done under absence (or near absence) of oxygen.
Main Difference – Combustion vs Oxidation Reactions of Ethanol
Ethanol is an alcohol having the molecular formula C2H5OH. The chemical formula of ethanol is CH3CH2OH. Ethanol is used as fuel since it can undergo combustion reactions. It can also undergo oxidation reactions to form aldehyde forms and carboxylic acid forms. The main difference between combustion and oxidation reactions of ethanol is that combustion reactions of ethanol always produce heat and light whereas oxidation reactions of ethanol do not always produce heat and light.
Key Areas Covered
1. What are the Combustion Reactions of Ethanol
– Definition, Properties, Reactions
2. What are the Oxidation Reactions of Ethanol
– Definition, Properties, Reactions
3. What is the Difference Between Combustion and Oxidation Reactions of Ethanol
– Comparison of Key Differences
Key Terms: Aldehyde, Biofuel, Carbon Dioxide, Carbon Monoxide, Carboxylic Acid, Combustion Reaction, Complete Combustion, Complete Oxidation, Ethanol, Gasoline, Incomplete Combustion, Incomplete Oxidation, Oxidation Reaction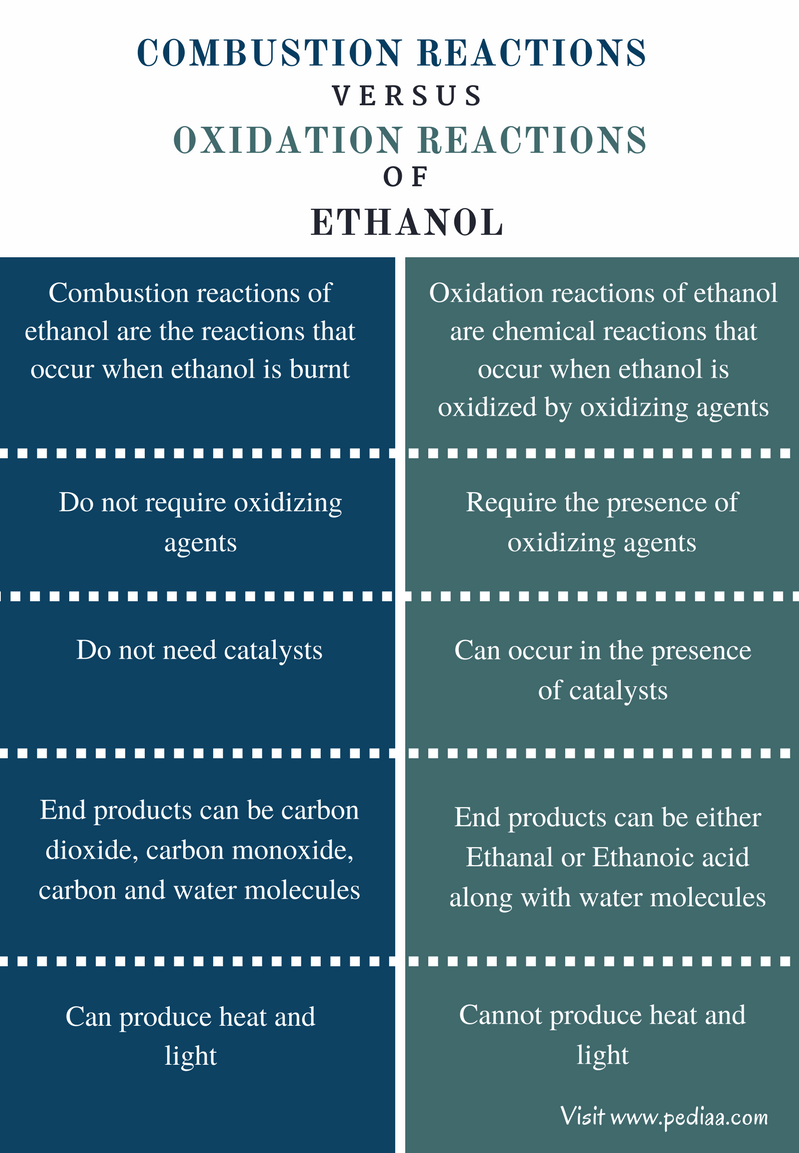
– Definition, Properties, Reactions
2. What are the Oxidation Reactions of Ethanol
– Definition, Properties, Reactions
3. What is the Difference Between Combustion and Oxidation Reactions of Ethanol
– Comparison of Key Differences

What are the Combustion Reactions of Ethanol
Combustion reactions of ethanol are the reactions that occur when ethanol is burnt. Ethanol is a highly flammable liquid that can be used as a fuel. Burning of ethanol can produce heat and light as energy. Therefore, combustion of ethanol is an exothermic reaction. When Ethanol is burnt in the presence of molecular oxygen (O2), it forms two final products. They are carbon dioxide (CO2) and water molecules (H2O).
The combustion of ethanol is indicated by a blue colored flame. The combustion of ethanol is a simple process which involves the combination of ethanol and oxygen. The combustion of ethanol can occur in two ways.
- Complete Combustion
- Incomplete Combustion

Figure 01: The blue flame indicates the complete combustion of ethanol.
The complete combustion results in CO2 and H2O. But incomplete combustion will produce carbon monoxide (CO) or Carbon (C) as a product. Incomplete combustion will take place when there is insufficient oxygen (O2).

Figure 01: The blue flame indicates the complete combustion of ethanol.
Complete Combustion of Ethanol
CH3CH2OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
Incomplete Combustion of Ethanol
CH3CH2OH(l) + 2O2(g) → 2CO(g) + 3H2O(l)
CH3CH2OH(l) + O2(g) → C(s) + 3H2O(l)
The incomplete combustion often results in a mixture of carbon monoxide (CO) gas and carbon (C) dust.
The heat produced by the combustion of ethanol is used to drive the piston of vehicle engines. Ethanol can also be used as a rocket fuel. In addition, ethanol can be produced as a biofuel from the biomass of plant material. Therefore, ethanol shows environmental friendly properties than fossil fuel.
Ethanol is a good additive for gasoline. The mixing of ethanol with gasoline prevents some emissions of air pollutants. However, when burnt, ethanol produces a flame which releases pollutants.
What are Oxidation Reactions of Ethanol
Oxidation reactions of ethanol are chemical reactions that take place when ethanol is oxidized by oxidizing agents. Oxidation of ethanol produces an aldehyde called Ethanal as the first product. It can undergo further oxidation to form the carboxylic acid form, which is known as Ethanoic acid.
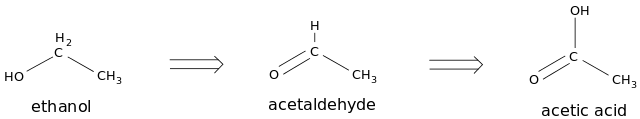
Figure 2: Oxidation of Ethanol
However, oxidation of ethanol can also occur in the presence of a catalyst. This catalyst is used to reduce the activation energy of the oxidation reaction. If the activation energy is high, the reaction will not be initiated. Oxidation can occur in two phases:
- Complete Oxidation
- Incomplete Oxidation
The complete oxidation of ethanol forms Ethanoic acid as the end product. Incomplete oxidation of ethanol forms Ethanal as the end product. Both oxidations will produce water molecules (H2O) as byproducts.

Figure 2: Oxidation of Ethanol
Complete Oxidation of Ethanol
Ethanol + Oxygen → Ethanal + Water → Ethanoic Acid + Water
CH3CH2OH(l) + [O] → CH3CHO(l) + H2O(l) → CH3COOH(l) + H2O(l)
The complete oxidation of ethanol results in Ethanoic acid at the end of the reaction. But ethanol oxidation first forms Ethanal and then Ethanal is further oxidized into Ethanoic acid.
Incomplete Oxidation of Ethanol
Ethanol + Oxygen → Ethanal + Water
CH3CH2OH(l) + [O] → CH3CHO(l) + H2O(l)
In the above equation, [O] indicates the atomic oxygen that comes from the oxidizing agent. As an example, let’s consider Sodium dichromate (Na2Cr2O7) was used as the oxidizing agent along with sulphuric acid (H2SO4).
CH3CH2OH(l) + Na2Cr2O7(aq) + H2SO4(aq) → CH3CHO(l) + 2NaCrO4(aq) + 2H2O(l)
Oxidation of Ethanol needs either a catalyst or an oxidizing agent in order to complete the reaction. However, oxidation of ethanol does not produce heat or light as energy forms. Another way of oxidation of ethanol is through catalysts. Silver catalyst is such a catalyst. Ethanol can be oxidized by passing a mixture of ethanol vapor and air over a silver catalyst at 500oC. This results in Ethanal as the product along with water (H2O).
Difference Between Combustion and Oxidation Reactions of Ethanol
Definition
Combustion Reactions of Ethanol: Combustion reactions of ethanol are the reactions that occur when ethanol is burnt.
Oxidation Reactions of Ethanol: Oxidation reactions of ethanol are chemical reactions that take place when ethanol is oxidized by oxidizing agents.
Oxidizing Agents
Combustion Reactions of Ethanol: Combustion reactions of ethanol do not require oxidizing agents.
Oxidation Reactions of Ethanol: Oxidation reactions of ethanol require the presence of oxidizing agents.
Catalysts
Combustion Reactions of Ethanol: Combustion reactions of ethanol do not need catalysts.
Oxidation Reactions of Ethanol: Oxidation reactions of ethanol can occur in the presence of catalysts.
End Products
Combustion Reactions of Ethanol: The end products of oxidation reactions of ethanol can be CO2, CO, C, and H2O.
Oxidation Reactions of Ethanol: The end products of combustion reactions of ethanol can be either Ethanal or Ethanoic acid along with H2O.
Energy Forms
Combustion Reactions of Ethanol: Combustion reactions of ethanol can produce heat and light.
Oxidation Reactions of Ethanol: Oxidation reactions of ethanol cannot produce heat and light.
Conclusion
Combustion is also an oxidation reaction since the end product of combustion is always an oxidized species. Moreover, combustion involves the combination of oxygen with the starting material. This also indicates that combustion is an oxidation reaction. Although there are similarities between these two phenomena, there are distinct properties that allow us to distinguish the difference between combustion and oxidation reactions of Ethanol.
Main Difference – Pure Substance vs Mixture
We come across a lot of substances such as water, fuel, food, beverages, and medicine during our daily activities. All these substances fall into two major categories. They are either pure substances or mixtures. The main difference between pure substance and mixture lies in their composition. A pure substance contains only one kind of compound. It can be the same molecule or atom. Mixtures are composed of several kinds of compounds. However, it’s important to look at them individually in order to understand the nature of these substances better.
This article explains,
1. What is a Pure Substance?
– Definition, Composition, Properties, Examples
2. What is a Mixture?
– Definition, Composition, Properties, Examples
3. What is the difference between Pure Substance and Mixture?
– Definition, Composition, Properties, Examples
– Definition, Composition, Properties, Examples

What is a Pure Substance
As mentioned above, a pure substance is composed of only one kind of substance. This cannot be separated into any other matter physically. The colour, texture, fragrance or taste of all the particles in a pure substance are the same. Elements and molecules are considered to be pure substances. They consist of only one component; hence, their physical and chemical properties are constant. Pure substances are of one phase and do not separate into two or more phases at a given temperature or pressure. They can be gas, liquid or solid.
Examples of pure substances
Gas:
H2 gas – composed only of H2 molecules. During normal pressure and temperature conditions, it exists in the gaseous phase. However, under extreme conditions, it tends to turn into a liquid.
Liquid:
Distilled deionized pure water- When water does not have any ions dissolved or any other substance dispersed, it is a pure substance in liquid form. However, water is often found naturally as a homogeneous mixture, with various other substances dissolved in it. Water is a liquid under normal conditions, but phase transfer happens when temperature and pressure are varied.
Solid:
Pure metals such as Gold, Silver, and Platinum are good examples for pure solid substances. These are often found as solids and can be turned into a molten liquid under high temperatures.
Pure substances have fixed melting points and boiling points, and this is very helpful in chemical synthesis to identify unknown substances. In organic chemistry, a melting point determination is very important to ensure that a certain desired chemical compound is isolated. The melting point of the unknown pure compound can be compared with that of known compounds to identify an unknown compound.


What is a Mixture
Mixtures consist of two or more substances which can be separated by physical or chemical means. The components of a mixture are not chemically bonded, but physical attractions may be present. This is the reason for their easy separation by processes like filtration, distillation, chromatography, extraction and centrifugation. Properties of components of a mixture are retained even after mixing up. Furthermore, when sugar is dissolved in water, we can still taste the sweet taste of sugar in the solution.
The component ratio is not fixed and may vary from mixture to mixture. Therefore properties like melting point and boiling point are not fixed either.
Mixtures are either homogeneous or heterogeneous depending on the uniformity of composition.
Homogeneous Mixtures
The composition is the same throughout the mixture. Particles are arranged in an orderly way. The components are of either atomic level or molecular level. Homogeneous mixtures are of one phase. There is no layer separation.
Heterogeneous Mixtures
Unlike homogeneous mixtures, there is no uniformity in the composition. Components can be of two phases (ex:- suspensions, soil). Furthermore, they can be distinguished by the naked eye. We can see grains of a rice and sand mixture separately and identify each component.
Read More:
Difference Between Homogeneous and Heterogeneous Mixtures

Difference Between Pure Substance and Mixture
Composition
Pure substance: Pure substances are made of only one matter; thus the composition is the same throughout.
Mixture: Mixtures are made up of several substances that are not chemically bonded.
Properties
Pure substance: Chemical and physical properties are constant.
Mixture: Chemical and physical properties may vary. Individual properties of components are retained.
Classification
Pure substance: Pure substances can be categorised as gas, liquid and solid.
Mixture: Mixtures are categorised as homogeneous and heterogeneous.
Examples
Pure substance: Examples include pure water, H2 gas, gold.
Mixture: Examples include sand and sugar, oil and water.
Finally, it can be stated that almost everything on the earth is either a mixture or a pure substance. A mixture can be defined as a collection of two or more pure substances.
Main Difference – Compound vs Mixture
A compound consists of different kind of atoms which are chemically bonded. A mixture is made up of two or more different kinds of substances (atoms, molecules or compounds) physically intermingled. The main difference between Compound and Mixture is that compounds are chemically bonded whereas mixtures are not.
This article explains,
1. What is a Compound?
– Definition, Characteristics, Types of Bonding, Examples
2. What is a Mixture?
– Definition, Characteristics, Examples
3. What is the difference between Compound and Mixture?
– Definition, Characteristics, Types of Bonding, Examples
– Definition, Characteristics, Examples

What is a Compound – Definition, Characteristics, Types of Bonding, Examples
Compounds are made of elements. A particular compound consists of two or more elements which are chemically bonded. A compound may be entirely different from the elements that they are made up of. For example, Na is a highly reactive metal, and Cl2 is a toxic gas. However, NaCl is a salt which is used for cooking. Another fine example is water which is made up of H2 and O2. Water is a liquid despite both its components being gases.
In certain compounds, the proportions of the content of the atom remain constant and are unique to that particular compound. If the proportion differs, it gives rise to a new compound. This scenario is elaborated by following examples.
Nitrogen and Oxygen give rise to these two compounds.
N2(g) + O2(g) → 2NO(g) Nitric Oxide or Nitrogen Oxide
2NO(g) + O2(g) → 2NO2(g) Nitrous Oxide or Nitrogen Dioxide
Although Nitrogen Oxide and Nitrous Dioxide are composed of the same elements, their compositions are different. Hence, it gives rise to two different compounds.
Compounds are formed when the attractive forces between the member atoms are greater than that of repulsive forces. Compounds are generally made by either covalent or ionic bonding. Energy is either taken in or given out while making compounds by chemical bonding.
Covalent Bonding
Electrons are shared by participating atoms as shown below. These kinds of compounds are called covalent compounds. If the two atoms are similarly attracted towards electrons (similar electronegativity), the compound is non-polar. However, if the electronegativity gap between the two atoms is huge, the compound becomes a polar one. A water molecule is the best example of this phenomena.
Non-polar compounds – Methane, Ammonia, Hexane
Polar Compounds – Water, CF, HF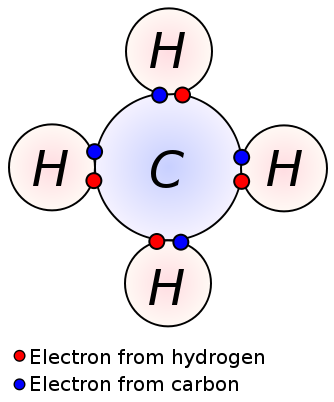

Ionic Bonding
Electrons are entirely transferred from one atom to the other. Hence, an electronic charge appears on both the atoms involved in the bonding. Compounds born from these bonds are mainly solids with high melting points and can conduct electrical current. Metal and non- metal elements partner up in forming these type of compounds.
Components of a compound cannot be physically separated. They can be only separated by chemical methods or electrolysis.
What is a Mixture – Definition, Characteristics, Types of Bonding
A mixture is a combination of two or more elements and/or compounds. Though these components are present together, neither are they bonded chemically nor do they make new substances. A good example is sand and water mixture where both components are not chemically bonded and can be separated as individual substances by filtration. Other physical means of separation are evaporation, distillation, chromatography, centrifugation and extraction. For these separation methods, physical properties of components in a mixture are considered. Some of these physical properties are density, size and solubility.
Components in compounds retain their individual characteristics. When you taste a salty water solution, you can feel the salty taste on your tongue. That indicates that the salt is able to give out its characteristic taste even when mixed with water. Mixtures do not have their own properties as compounds do.
Mixtures are often homogeneous or heterogeneous.
Homogeneous mixtures – The composition of the mixture is the same throughout.
Ex:- Salt dissolved in water
Heterogeneous mixtures – Composition may vary from point to point in the mixture.
Ex:- Smog
The air we breathe, and the vast blue ocean can be considered as the largest mixtures found on earth. Both are heterogeneous mixtures.


Difference Between Compound and Mixture
Bond
Compounds: Components are chemically bonded.
Ex:- NaCl, H2O
Mixtures: Components are not chemically bonded.
Ex:- Salty Water, Sand and Sugar
Separation
Compounds: Components cannot be physically separated. They can be separated through electrolysis.
Mixtures: Components can be physically separated easily through methods such as filtration, chromatography, centrifugation, dialysis, evaporation, and distillation.
Characteristics
Compounds: Compounds show their own characteristics, not the individual features of components.
Mixtures: Compounds do not show their own characteristics. Individual features of components are displayed.
Ratio
Compounds: Ratio of components is fixed.
Mixtures: Ratio of components may vary.
Boiling Point and Melting Point
Compounds: Boiling point and melting point are constant for a particular compound.
Mixtures: Boiling point and melting point are not constant.
Energy Transfer
Compounds: Energy is given out or in to prepare compounds through chemical bonding.
Mixtures: There is no or little energy transfer.
Categories
Compounds: Compounds can be covalent or ionic.
Mixtures: Mixtures can be homogeneous or heterogeneous.
Main Difference – Compound vs Mixture
A compound consists of different kind of atoms which are chemically bonded. A mixture is made up of two or more different kinds of substances (atoms, molecules or compounds) physically intermingled. The main difference between Compound and Mixture is that compounds are chemically bonded whereas mixtures are not.
This article explains,
1. What is a Compound?
– Definition, Characteristics, Types of Bonding, Examples
2. What is a Mixture?
– Definition, Characteristics, Examples
3. What is the difference between Compound and Mixture?
– Definition, Characteristics, Types of Bonding, Examples
– Definition, Characteristics, Examples

What is a Compound – Definition, Characteristics, Types of Bonding, Examples
Compounds are made of elements. A particular compound consists of two or more elements which are chemically bonded. A compound may be entirely different from the elements that they are made up of. For example, Na is a highly reactive metal, and Cl2 is a toxic gas. However, NaCl is a salt which is used for cooking. Another fine example is water which is made up of H2 and O2. Water is a liquid despite both its components being gases.
In certain compounds, the proportions of the content of the atom remain constant and are unique to that particular compound. If the proportion differs, it gives rise to a new compound. This scenario is elaborated by following examples.
Nitrogen and Oxygen give rise to these two compounds.
N2(g) + O2(g) → 2NO(g) Nitric Oxide or Nitrogen Oxide
2NO(g) + O2(g) → 2NO2(g) Nitrous Oxide or Nitrogen Dioxide
Although Nitrogen Oxide and Nitrous Dioxide are composed of the same elements, their compositions are different. Hence, it gives rise to two different compounds.
Compounds are formed when the attractive forces between the member atoms are greater than that of repulsive forces. Compounds are generally made by either covalent or ionic bonding. Energy is either taken in or given out while making compounds by chemical bonding.
Covalent Bonding
Electrons are shared by participating atoms as shown below. These kinds of compounds are called covalent compounds. If the two atoms are similarly attracted towards electrons (similar electronegativity), the compound is non-polar. However, if the electronegativity gap between the two atoms is huge, the compound becomes a polar one. A water molecule is the best example of this phenomena.
Non-polar compounds – Methane, Ammonia, Hexane
Polar Compounds – Water, CF, HF

Ionic Bonding
Electrons are entirely transferred from one atom to the other. Hence, an electronic charge appears on both the atoms involved in the bonding. Compounds born from these bonds are mainly solids with high melting points and can conduct electrical current. Metal and non- metal elements partner up in forming these type of compounds.
Components of a compound cannot be physically separated. They can be only separated by chemical methods or electrolysis.
What is a Mixture – Definition, Characteristics, Types of Bonding
A mixture is a combination of two or more elements and/or compounds. Though these components are present together, neither are they bonded chemically nor do they make new substances. A good example is sand and water mixture where both components are not chemically bonded and can be separated as individual substances by filtration. Other physical means of separation are evaporation, distillation, chromatography, centrifugation and extraction. For these separation methods, physical properties of components in a mixture are considered. Some of these physical properties are density, size and solubility.
Components in compounds retain their individual characteristics. When you taste a salty water solution, you can feel the salty taste on your tongue. That indicates that the salt is able to give out its characteristic taste even when mixed with water. Mixtures do not have their own properties as compounds do.
Mixtures are often homogeneous or heterogeneous.
Homogeneous mixtures – The composition of the mixture is the same throughout.
Ex:- Salt dissolved in water
Heterogeneous mixtures – Composition may vary from point to point in the mixture.
Ex:- Smog
The air we breathe, and the vast blue ocean can be considered as the largest mixtures found on earth. Both are heterogeneous mixtures.


Difference Between Compound and Mixture
Bond
Compounds: Components are chemically bonded.
Ex:- NaCl, H2O
Mixtures: Components are not chemically bonded.
Ex:- Salty Water, Sand and Sugar
Separation
Compounds: Components cannot be physically separated. They can be separated through electrolysis.
Mixtures: Components can be physically separated easily through methods such as filtration, chromatography, centrifugation, dialysis, evaporation, and distillation.
Characteristics
Compounds: Compounds show their own characteristics, not the individual features of components.
Mixtures: Compounds do not show their own characteristics. Individual features of components are displayed.
Ratio
Compounds: Ratio of components is fixed.
Mixtures: Ratio of components may vary.
Boiling Point and Melting Point
Compounds: Boiling point and melting point are constant for a particular compound.
Mixtures: Boiling point and melting point are not constant.
Energy Transfer
Compounds: Energy is given out or in to prepare compounds through chemical bonding.
Mixtures: There is no or little energy transfer.
Categories
Compounds: Compounds can be covalent or ionic.
Mixtures: Mixtures can be homogeneous or heterogeneous.
What is the Difference Between Gas and Liquid Chromatography
The main difference between gas and liquid chromatography is that the mobile phase of gas chromatography is a gas, which is most often helium, whereas the mobile phase of liquid chromatography is a liquid, which can be either polar or non-polar. Furthermore, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica. Moreover, gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas and liquid chromatography are the two types of chromatography techniques classified based on the physical state of the mobile phase. Generally, the mobile phase is the phase that flows through the stationary phase.
The main difference between gas and liquid chromatography is that the mobile phase of gas chromatography is a gas, which is most often helium, whereas the mobile phase of liquid chromatography is a liquid, which can be either polar or non-polar. Furthermore, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica. Moreover, gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas and liquid chromatography are the two types of chromatography techniques classified based on the physical state of the mobile phase. Generally, the mobile phase is the phase that flows through the stationary phase.
Key Areas Covered
1. What is Gas Chromatography
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
1. What is Gas Chromatography
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
Key Terms
Column Chromatography, Gas Chromatography, Liquid Chromatography, Mobile Phase, Stationary Phase
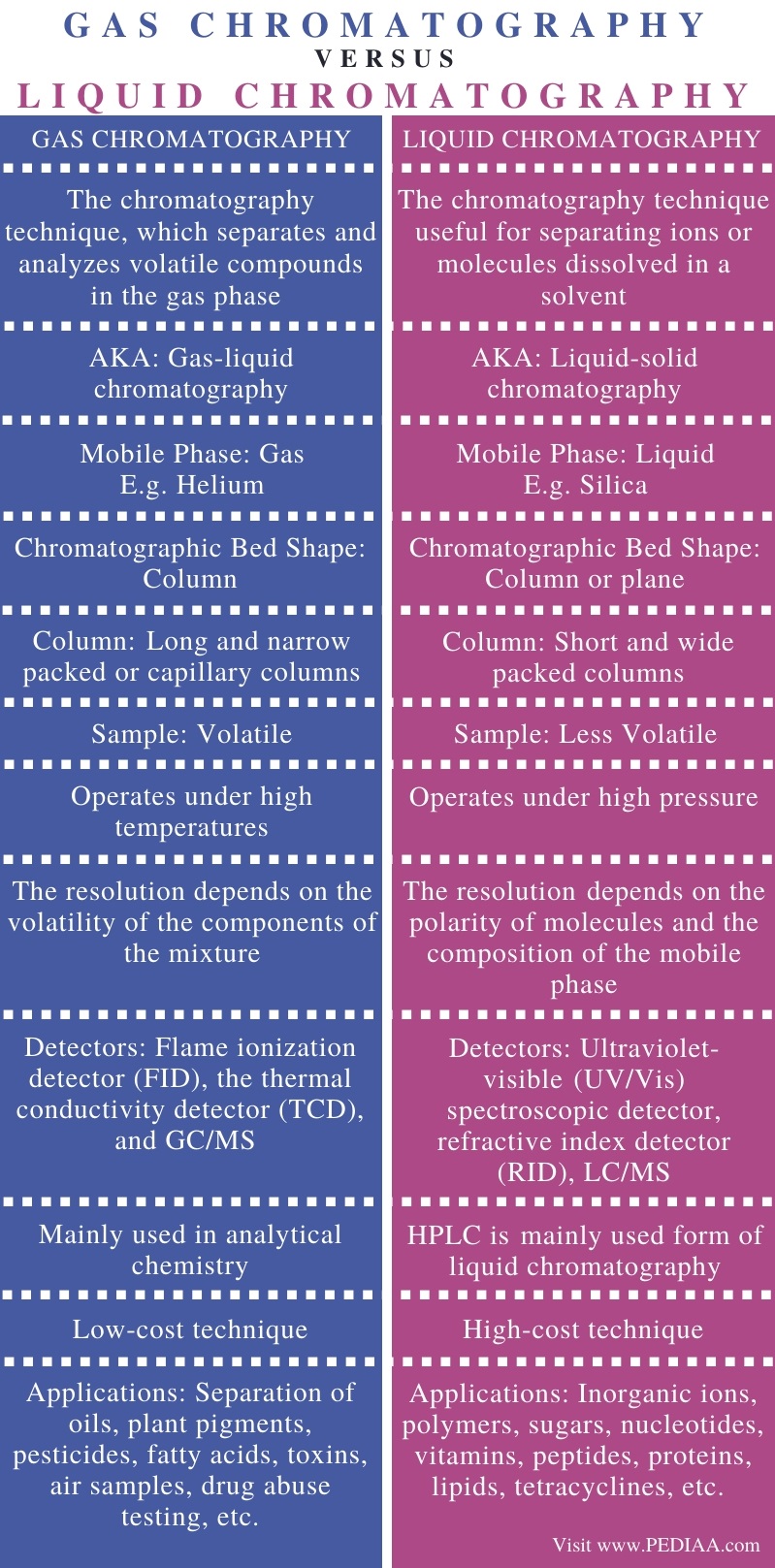
Column Chromatography, Gas Chromatography, Liquid Chromatography, Mobile Phase, Stationary Phase

What is Gas Chromatography
Gas chromatography is the type of analytical chromatography whose mobile phase is a gas. Generally, this carrier gas is either an inert gas such as helium or a non-reactive gas such as nitrogen. However, hydrogen is preferred over helium for better separation, although helium is the common carrier gas in 90% of the instruments. Moreover, the stationary phase of gas chromatography is a liquid. Therefore, the full name for gas chromatography is gas-liquid chromatography. Here, a microscopic layer of liquid stationary phase occurs on inert solid support inside a tiny glass tube. Thus, gas chromatography operates as a column chromatography technique.
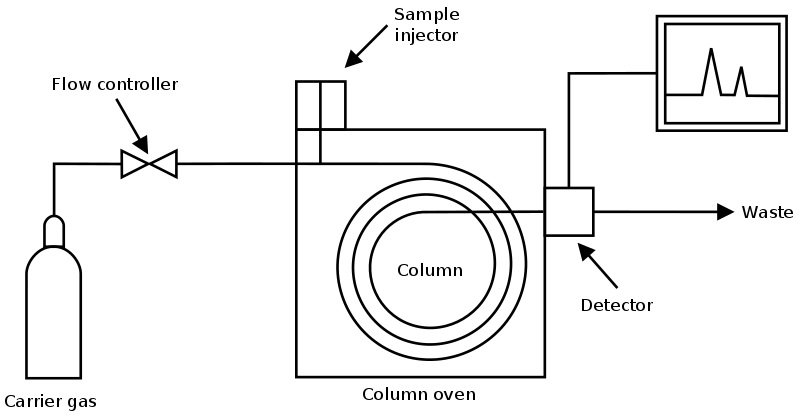
Figure 1: Gas Chromatography
Furthermore, gas chromatography is responsible for analyzing compounds in the form of vapor. Also, its separation of compounds depends on the partition equilibrium of components between the mobile and the stationary phase. However, the usage of high temperature in gas chromatography makes it unsuitable for separating polymers of high molecular weights. Basically, this is due to the inability of these polymers to become a vapor. In preparative chromatography, gas chromatography is an important tool to prepare pure components from a mixture.
Gas chromatography is the type of analytical chromatography whose mobile phase is a gas. Generally, this carrier gas is either an inert gas such as helium or a non-reactive gas such as nitrogen. However, hydrogen is preferred over helium for better separation, although helium is the common carrier gas in 90% of the instruments. Moreover, the stationary phase of gas chromatography is a liquid. Therefore, the full name for gas chromatography is gas-liquid chromatography. Here, a microscopic layer of liquid stationary phase occurs on inert solid support inside a tiny glass tube. Thus, gas chromatography operates as a column chromatography technique.

Figure 1: Gas Chromatography
Furthermore, gas chromatography is responsible for analyzing compounds in the form of vapor. Also, its separation of compounds depends on the partition equilibrium of components between the mobile and the stationary phase. However, the usage of high temperature in gas chromatography makes it unsuitable for separating polymers of high molecular weights. Basically, this is due to the inability of these polymers to become a vapor. In preparative chromatography, gas chromatography is an important tool to prepare pure components from a mixture.
What is Liquid Chromatography
Liquid chromatography is the other type of chromatography classified based on the physical state of the mobile phase. Significantly, its mobile phase is a liquid. For instance, the stationary phase of the liquid chromatography is solid. Therefore, the basic chromatography structure can be either a column or plane chromatography. Generally, in column chromatography, the stationary bed occurs within a tube. In contrast, in planar chromatography, the stationary phase occurs on a plane.
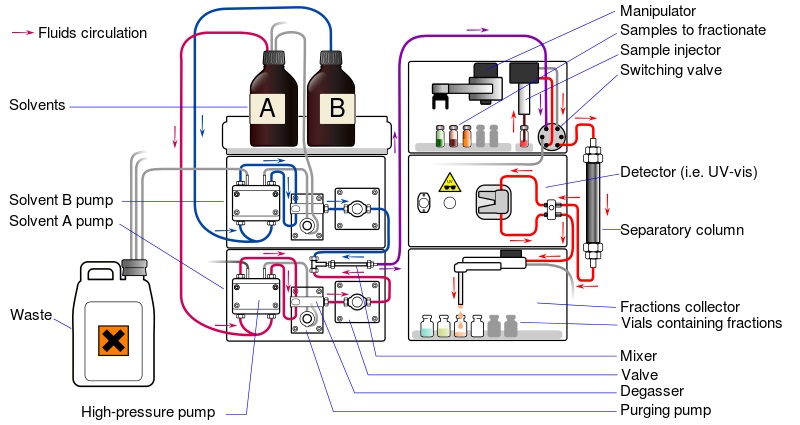
Figure 2: Liquid Chromatography
Moreover, present-day liquid chromatography is mainly the high-performance liquid chromatography (HPLC), which uses a very small packing of articles. Also, HLC operates under high pressure. Therefore, the stationary phase is mainly a porous membrane or a porous monolithic layer, composed of spherical or irregular shaped particles. Meanwhile, the liquid mobile phase flows on the stationary phase under high pressure. However, there are two types of HPLC techniques according to the polarity of the mobile and stationary phases. They are the normal phase and reverse-phase liquid chromatography. Typically, in the normal phase liquid chromatography, the mobile phase is non-polar (e.g. toluene) while the stationary phase is polar (e.g. silica). On the other hand, in reverse-phase liquid chromatography, the mobile phase is polar (e.g. water-methanol mixture) while the stationary phase is non-polar (e.g. C18). However, both types of HPLC operate under room temperature.
Liquid chromatography is the other type of chromatography classified based on the physical state of the mobile phase. Significantly, its mobile phase is a liquid. For instance, the stationary phase of the liquid chromatography is solid. Therefore, the basic chromatography structure can be either a column or plane chromatography. Generally, in column chromatography, the stationary bed occurs within a tube. In contrast, in planar chromatography, the stationary phase occurs on a plane.

Figure 2: Liquid Chromatography
Moreover, present-day liquid chromatography is mainly the high-performance liquid chromatography (HPLC), which uses a very small packing of articles. Also, HLC operates under high pressure. Therefore, the stationary phase is mainly a porous membrane or a porous monolithic layer, composed of spherical or irregular shaped particles. Meanwhile, the liquid mobile phase flows on the stationary phase under high pressure. However, there are two types of HPLC techniques according to the polarity of the mobile and stationary phases. They are the normal phase and reverse-phase liquid chromatography. Typically, in the normal phase liquid chromatography, the mobile phase is non-polar (e.g. toluene) while the stationary phase is polar (e.g. silica). On the other hand, in reverse-phase liquid chromatography, the mobile phase is polar (e.g. water-methanol mixture) while the stationary phase is non-polar (e.g. C18). However, both types of HPLC operate under room temperature.
Similarities Between Gas and Liquid Chromatography
- Gas and liquid chromatography are the two types of chromatography techniques classified according to the type of mobile phase.
- Both are laboratory techniques for the separation of a mixture. Also, both are analytical separation methods.
- Generally, the mixture to be separated is dissolved in the mobile phase, which carries it through the stationary phase.
- However, the separation occurs depending on the properties of the components of the mixture, determining the variable interactions towards the mobile or stationary phase.
- Both can be column chromatography.
- Mass spectrometry (MS) is the most powerful detection method for both types of chromatography.
- Gas and liquid chromatography are the two types of chromatography techniques classified according to the type of mobile phase.
- Both are laboratory techniques for the separation of a mixture. Also, both are analytical separation methods.
- Generally, the mixture to be separated is dissolved in the mobile phase, which carries it through the stationary phase.
- However, the separation occurs depending on the properties of the components of the mixture, determining the variable interactions towards the mobile or stationary phase.
- Both can be column chromatography.
- Mass spectrometry (MS) is the most powerful detection method for both types of chromatography.
Difference Between Gas and Liquid Chromatography
Definition
Gas chromatography refers to the chromatography technique which separates and analyzes volatile compounds in the gas phase while liquid chromatography refers to the chromatography technique useful for separating ions or molecules dissolved in a solvent.
Gas chromatography refers to the chromatography technique which separates and analyzes volatile compounds in the gas phase while liquid chromatography refers to the chromatography technique useful for separating ions or molecules dissolved in a solvent.
Also Known as
Another name for gas chromatography is gas-liquid chromatography while another name for liquid chromatography is liquid-solid chromatography.
Another name for gas chromatography is gas-liquid chromatography while another name for liquid chromatography is liquid-solid chromatography.
Type of Mobile Phase
The mobile phase of gas chromatography is a gas while the mobile phase of liquid chromatography is a liquid.
The mobile phase of gas chromatography is a gas while the mobile phase of liquid chromatography is a liquid.
Examples
The mobile phase of gas chromatography is most often helium, while the mobile phase of liquid chromatography can be either polar or non-polar.
The mobile phase of gas chromatography is most often helium, while the mobile phase of liquid chromatography can be either polar or non-polar.
Mobile Phase Gradient
While the mobile phase has no gradient in gas chromatography, the mobile phase has a gradient in liquid chromatography.
While the mobile phase has no gradient in gas chromatography, the mobile phase has a gradient in liquid chromatography.
Stationary Phase
Moreover, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica.
Moreover, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica.
Chromatographic Bed Shape
Gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Columns
Long and narrow packed or capillary columns are used in gas chromatography while short and wide packed columns are used in liquid chromatography.
Long and narrow packed or capillary columns are used in gas chromatography while short and wide packed columns are used in liquid chromatography.
Sample
Components of the sample are volatile in gas chromatography, while the components of the sample are less volatile.
Components of the sample are volatile in gas chromatography, while the components of the sample are less volatile.
Chromatographic Conditions
Gas chromatography operates under high temperatures while liquid chromatography operates under high pressure.
Gas chromatography operates under high temperatures while liquid chromatography operates under high pressure.
Resolution
The resolution of gas chromatography depends on the volatility of the components of the mixture while the resolution of liquid chromatography depends on the polarity of molecules and the composition of the mobile phase.
The resolution of gas chromatography depends on the volatility of the components of the mixture while the resolution of liquid chromatography depends on the polarity of molecules and the composition of the mobile phase.
Detectors
The two main types of detectors used in gas chromatography are flame ionization detector (FID) and thermal conductivity detector (TCD) while the two main types of detectors used in liquid chromatography are ultraviolet-visible (UV/Vis) spectroscopic detector and refractive index detector (RID).
The two main types of detectors used in gas chromatography are flame ionization detector (FID) and thermal conductivity detector (TCD) while the two main types of detectors used in liquid chromatography are ultraviolet-visible (UV/Vis) spectroscopic detector and refractive index detector (RID).
Importance
Gas chromatography is mainly used in analytical chemistry while high-performance liquid chromatography is the mainly used form of liquid chromatography.
Gas chromatography is mainly used in analytical chemistry while high-performance liquid chromatography is the mainly used form of liquid chromatography.
Relative Cost
Also, gas chromatography is a low-cost technique, while liquid chromatography is a high-cost technique.
Also, gas chromatography is a low-cost technique, while liquid chromatography is a high-cost technique.
Applications
Gas chromatography is used for the separation of oils, plant pigments, pesticides, fatty acids, toxins, air samples, drug abuse testing, etc. while liquid chromatography is used for inorganic ions, polymers, sugars, nucleotides, vitamins, peptides, proteins, lipids, tetracyclines, etc.
Gas chromatography is used for the separation of oils, plant pigments, pesticides, fatty acids, toxins, air samples, drug abuse testing, etc. while liquid chromatography is used for inorganic ions, polymers, sugars, nucleotides, vitamins, peptides, proteins, lipids, tetracyclines, etc.
Conclusion
Gas chromatography is the type of chromatography, using a gas mobile phase. Generally, the mobile phase is helium. Also, the stationary phase of gas chromatography is a liquid with a silicone base. Therefore, it is a type of column chromatography. Liquid chromatography is another type of chromatography, using a liquid mobile phase, which is mainly silica. Moreover, liquid chromatography can be either column or plane chromatography. Hence, the main difference between gas and liquid chromatography is the physical state of the mobile phase.
Gas chromatography is the type of chromatography, using a gas mobile phase. Generally, the mobile phase is helium. Also, the stationary phase of gas chromatography is a liquid with a silicone base. Therefore, it is a type of column chromatography. Liquid chromatography is another type of chromatography, using a liquid mobile phase, which is mainly silica. Moreover, liquid chromatography can be either column or plane chromatography. Hence, the main difference between gas and liquid chromatography is the physical state of the mobile phase.
What is the Difference Between Gas and Liquid Chromatography
The main difference between gas and liquid chromatography is that the mobile phase of gas chromatography is a gas, which is most often helium, whereas the mobile phase of liquid chromatography is a liquid, which can be either polar or non-polar. Furthermore, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica. Moreover, gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas and liquid chromatography are the two types of chromatography techniques classified based on the physical state of the mobile phase. Generally, the mobile phase is the phase that flows through the stationary phase.
The main difference between gas and liquid chromatography is that the mobile phase of gas chromatography is a gas, which is most often helium, whereas the mobile phase of liquid chromatography is a liquid, which can be either polar or non-polar. Furthermore, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica. Moreover, gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas and liquid chromatography are the two types of chromatography techniques classified based on the physical state of the mobile phase. Generally, the mobile phase is the phase that flows through the stationary phase.
Key Areas Covered
1. What is Gas Chromatography
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
1. What is Gas Chromatography
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
Key Terms
Column Chromatography, Gas Chromatography, Liquid Chromatography, Mobile Phase, Stationary Phase

Column Chromatography, Gas Chromatography, Liquid Chromatography, Mobile Phase, Stationary Phase

What is Gas Chromatography
Gas chromatography is the type of analytical chromatography whose mobile phase is a gas. Generally, this carrier gas is either an inert gas such as helium or a non-reactive gas such as nitrogen. However, hydrogen is preferred over helium for better separation, although helium is the common carrier gas in 90% of the instruments. Moreover, the stationary phase of gas chromatography is a liquid. Therefore, the full name for gas chromatography is gas-liquid chromatography. Here, a microscopic layer of liquid stationary phase occurs on inert solid support inside a tiny glass tube. Thus, gas chromatography operates as a column chromatography technique.

Figure 1: Gas Chromatography
Furthermore, gas chromatography is responsible for analyzing compounds in the form of vapor. Also, its separation of compounds depends on the partition equilibrium of components between the mobile and the stationary phase. However, the usage of high temperature in gas chromatography makes it unsuitable for separating polymers of high molecular weights. Basically, this is due to the inability of these polymers to become a vapor. In preparative chromatography, gas chromatography is an important tool to prepare pure components from a mixture.
Gas chromatography is the type of analytical chromatography whose mobile phase is a gas. Generally, this carrier gas is either an inert gas such as helium or a non-reactive gas such as nitrogen. However, hydrogen is preferred over helium for better separation, although helium is the common carrier gas in 90% of the instruments. Moreover, the stationary phase of gas chromatography is a liquid. Therefore, the full name for gas chromatography is gas-liquid chromatography. Here, a microscopic layer of liquid stationary phase occurs on inert solid support inside a tiny glass tube. Thus, gas chromatography operates as a column chromatography technique.

Figure 1: Gas Chromatography
Furthermore, gas chromatography is responsible for analyzing compounds in the form of vapor. Also, its separation of compounds depends on the partition equilibrium of components between the mobile and the stationary phase. However, the usage of high temperature in gas chromatography makes it unsuitable for separating polymers of high molecular weights. Basically, this is due to the inability of these polymers to become a vapor. In preparative chromatography, gas chromatography is an important tool to prepare pure components from a mixture.
What is Liquid Chromatography
Liquid chromatography is the other type of chromatography classified based on the physical state of the mobile phase. Significantly, its mobile phase is a liquid. For instance, the stationary phase of the liquid chromatography is solid. Therefore, the basic chromatography structure can be either a column or plane chromatography. Generally, in column chromatography, the stationary bed occurs within a tube. In contrast, in planar chromatography, the stationary phase occurs on a plane.

Figure 2: Liquid Chromatography
Moreover, present-day liquid chromatography is mainly the high-performance liquid chromatography (HPLC), which uses a very small packing of articles. Also, HLC operates under high pressure. Therefore, the stationary phase is mainly a porous membrane or a porous monolithic layer, composed of spherical or irregular shaped particles. Meanwhile, the liquid mobile phase flows on the stationary phase under high pressure. However, there are two types of HPLC techniques according to the polarity of the mobile and stationary phases. They are the normal phase and reverse-phase liquid chromatography. Typically, in the normal phase liquid chromatography, the mobile phase is non-polar (e.g. toluene) while the stationary phase is polar (e.g. silica). On the other hand, in reverse-phase liquid chromatography, the mobile phase is polar (e.g. water-methanol mixture) while the stationary phase is non-polar (e.g. C18). However, both types of HPLC operate under room temperature.
Liquid chromatography is the other type of chromatography classified based on the physical state of the mobile phase. Significantly, its mobile phase is a liquid. For instance, the stationary phase of the liquid chromatography is solid. Therefore, the basic chromatography structure can be either a column or plane chromatography. Generally, in column chromatography, the stationary bed occurs within a tube. In contrast, in planar chromatography, the stationary phase occurs on a plane.

Figure 2: Liquid Chromatography
Moreover, present-day liquid chromatography is mainly the high-performance liquid chromatography (HPLC), which uses a very small packing of articles. Also, HLC operates under high pressure. Therefore, the stationary phase is mainly a porous membrane or a porous monolithic layer, composed of spherical or irregular shaped particles. Meanwhile, the liquid mobile phase flows on the stationary phase under high pressure. However, there are two types of HPLC techniques according to the polarity of the mobile and stationary phases. They are the normal phase and reverse-phase liquid chromatography. Typically, in the normal phase liquid chromatography, the mobile phase is non-polar (e.g. toluene) while the stationary phase is polar (e.g. silica). On the other hand, in reverse-phase liquid chromatography, the mobile phase is polar (e.g. water-methanol mixture) while the stationary phase is non-polar (e.g. C18). However, both types of HPLC operate under room temperature.
Similarities Between Gas and Liquid Chromatography
- Gas and liquid chromatography are the two types of chromatography techniques classified according to the type of mobile phase.
- Both are laboratory techniques for the separation of a mixture. Also, both are analytical separation methods.
- Generally, the mixture to be separated is dissolved in the mobile phase, which carries it through the stationary phase.
- However, the separation occurs depending on the properties of the components of the mixture, determining the variable interactions towards the mobile or stationary phase.
- Both can be column chromatography.
- Mass spectrometry (MS) is the most powerful detection method for both types of chromatography.
- Gas and liquid chromatography are the two types of chromatography techniques classified according to the type of mobile phase.
- Both are laboratory techniques for the separation of a mixture. Also, both are analytical separation methods.
- Generally, the mixture to be separated is dissolved in the mobile phase, which carries it through the stationary phase.
- However, the separation occurs depending on the properties of the components of the mixture, determining the variable interactions towards the mobile or stationary phase.
- Both can be column chromatography.
- Mass spectrometry (MS) is the most powerful detection method for both types of chromatography.
Difference Between Gas and Liquid Chromatography
Definition
Gas chromatography refers to the chromatography technique which separates and analyzes volatile compounds in the gas phase while liquid chromatography refers to the chromatography technique useful for separating ions or molecules dissolved in a solvent.
Gas chromatography refers to the chromatography technique which separates and analyzes volatile compounds in the gas phase while liquid chromatography refers to the chromatography technique useful for separating ions or molecules dissolved in a solvent.
Also Known as
Another name for gas chromatography is gas-liquid chromatography while another name for liquid chromatography is liquid-solid chromatography.
Another name for gas chromatography is gas-liquid chromatography while another name for liquid chromatography is liquid-solid chromatography.
Type of Mobile Phase
The mobile phase of gas chromatography is a gas while the mobile phase of liquid chromatography is a liquid.
The mobile phase of gas chromatography is a gas while the mobile phase of liquid chromatography is a liquid.
Examples
The mobile phase of gas chromatography is most often helium, while the mobile phase of liquid chromatography can be either polar or non-polar.
The mobile phase of gas chromatography is most often helium, while the mobile phase of liquid chromatography can be either polar or non-polar.
Mobile Phase Gradient
While the mobile phase has no gradient in gas chromatography, the mobile phase has a gradient in liquid chromatography.
While the mobile phase has no gradient in gas chromatography, the mobile phase has a gradient in liquid chromatography.
Stationary Phase
Moreover, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica.
Moreover, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica.
Chromatographic Bed Shape
Gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Columns
Long and narrow packed or capillary columns are used in gas chromatography while short and wide packed columns are used in liquid chromatography.
Long and narrow packed or capillary columns are used in gas chromatography while short and wide packed columns are used in liquid chromatography.
Sample
Components of the sample are volatile in gas chromatography, while the components of the sample are less volatile.
Components of the sample are volatile in gas chromatography, while the components of the sample are less volatile.
Chromatographic Conditions
Gas chromatography operates under high temperatures while liquid chromatography operates under high pressure.
Gas chromatography operates under high temperatures while liquid chromatography operates under high pressure.
Resolution
The resolution of gas chromatography depends on the volatility of the components of the mixture while the resolution of liquid chromatography depends on the polarity of molecules and the composition of the mobile phase.
The resolution of gas chromatography depends on the volatility of the components of the mixture while the resolution of liquid chromatography depends on the polarity of molecules and the composition of the mobile phase.
Detectors
The two main types of detectors used in gas chromatography are flame ionization detector (FID) and thermal conductivity detector (TCD) while the two main types of detectors used in liquid chromatography are ultraviolet-visible (UV/Vis) spectroscopic detector and refractive index detector (RID).
The two main types of detectors used in gas chromatography are flame ionization detector (FID) and thermal conductivity detector (TCD) while the two main types of detectors used in liquid chromatography are ultraviolet-visible (UV/Vis) spectroscopic detector and refractive index detector (RID).
Importance
Gas chromatography is mainly used in analytical chemistry while high-performance liquid chromatography is the mainly used form of liquid chromatography.
Gas chromatography is mainly used in analytical chemistry while high-performance liquid chromatography is the mainly used form of liquid chromatography.
Relative Cost
Also, gas chromatography is a low-cost technique, while liquid chromatography is a high-cost technique.
Also, gas chromatography is a low-cost technique, while liquid chromatography is a high-cost technique.
Applications
Gas chromatography is used for the separation of oils, plant pigments, pesticides, fatty acids, toxins, air samples, drug abuse testing, etc. while liquid chromatography is used for inorganic ions, polymers, sugars, nucleotides, vitamins, peptides, proteins, lipids, tetracyclines, etc.
Gas chromatography is used for the separation of oils, plant pigments, pesticides, fatty acids, toxins, air samples, drug abuse testing, etc. while liquid chromatography is used for inorganic ions, polymers, sugars, nucleotides, vitamins, peptides, proteins, lipids, tetracyclines, etc.
Conclusion
Gas chromatography is the type of chromatography, using a gas mobile phase. Generally, the mobile phase is helium. Also, the stationary phase of gas chromatography is a liquid with a silicone base. Therefore, it is a type of column chromatography. Liquid chromatography is another type of chromatography, using a liquid mobile phase, which is mainly silica. Moreover, liquid chromatography can be either column or plane chromatography. Hence, the main difference between gas and liquid chromatography is the physical state of the mobile phase.
Gas chromatography is the type of chromatography, using a gas mobile phase. Generally, the mobile phase is helium. Also, the stationary phase of gas chromatography is a liquid with a silicone base. Therefore, it is a type of column chromatography. Liquid chromatography is another type of chromatography, using a liquid mobile phase, which is mainly silica. Moreover, liquid chromatography can be either column or plane chromatography. Hence, the main difference between gas and liquid chromatography is the physical state of the mobile phase.
What is the Difference Between Gas and Liquid Chromatography
The main difference between gas and liquid chromatography is that the mobile phase of gas chromatography is a gas, which is most often helium, whereas the mobile phase of liquid chromatography is a liquid, which can be either polar or non-polar. Furthermore, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica. Moreover, gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas and liquid chromatography are the two types of chromatography techniques classified based on the physical state of the mobile phase. Generally, the mobile phase is the phase that flows through the stationary phase.
The main difference between gas and liquid chromatography is that the mobile phase of gas chromatography is a gas, which is most often helium, whereas the mobile phase of liquid chromatography is a liquid, which can be either polar or non-polar. Furthermore, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica. Moreover, gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas and liquid chromatography are the two types of chromatography techniques classified based on the physical state of the mobile phase. Generally, the mobile phase is the phase that flows through the stationary phase.
Key Areas Covered
1. What is Gas Chromatography
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
1. What is Gas Chromatography
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
– Definition, Principle, Importance
2. What is Liquid Chromatography
– Definition, Principle, Importance
3. What are the Similarities Between Gas and Liquid Chromatography
– Outline of Common Features
4. What is the Difference Between Gas and Liquid Chromatography
– Comparison of Key Differences
Key Terms
Column Chromatography, Gas Chromatography, Liquid Chromatography, Mobile Phase, Stationary Phase

Column Chromatography, Gas Chromatography, Liquid Chromatography, Mobile Phase, Stationary Phase

What is Gas Chromatography
Gas chromatography is the type of analytical chromatography whose mobile phase is a gas. Generally, this carrier gas is either an inert gas such as helium or a non-reactive gas such as nitrogen. However, hydrogen is preferred over helium for better separation, although helium is the common carrier gas in 90% of the instruments. Moreover, the stationary phase of gas chromatography is a liquid. Therefore, the full name for gas chromatography is gas-liquid chromatography. Here, a microscopic layer of liquid stationary phase occurs on inert solid support inside a tiny glass tube. Thus, gas chromatography operates as a column chromatography technique.

Figure 1: Gas Chromatography
Furthermore, gas chromatography is responsible for analyzing compounds in the form of vapor. Also, its separation of compounds depends on the partition equilibrium of components between the mobile and the stationary phase. However, the usage of high temperature in gas chromatography makes it unsuitable for separating polymers of high molecular weights. Basically, this is due to the inability of these polymers to become a vapor. In preparative chromatography, gas chromatography is an important tool to prepare pure components from a mixture.
Gas chromatography is the type of analytical chromatography whose mobile phase is a gas. Generally, this carrier gas is either an inert gas such as helium or a non-reactive gas such as nitrogen. However, hydrogen is preferred over helium for better separation, although helium is the common carrier gas in 90% of the instruments. Moreover, the stationary phase of gas chromatography is a liquid. Therefore, the full name for gas chromatography is gas-liquid chromatography. Here, a microscopic layer of liquid stationary phase occurs on inert solid support inside a tiny glass tube. Thus, gas chromatography operates as a column chromatography technique.

Figure 1: Gas Chromatography
Furthermore, gas chromatography is responsible for analyzing compounds in the form of vapor. Also, its separation of compounds depends on the partition equilibrium of components between the mobile and the stationary phase. However, the usage of high temperature in gas chromatography makes it unsuitable for separating polymers of high molecular weights. Basically, this is due to the inability of these polymers to become a vapor. In preparative chromatography, gas chromatography is an important tool to prepare pure components from a mixture.
What is Liquid Chromatography
Liquid chromatography is the other type of chromatography classified based on the physical state of the mobile phase. Significantly, its mobile phase is a liquid. For instance, the stationary phase of the liquid chromatography is solid. Therefore, the basic chromatography structure can be either a column or plane chromatography. Generally, in column chromatography, the stationary bed occurs within a tube. In contrast, in planar chromatography, the stationary phase occurs on a plane.

Figure 2: Liquid Chromatography
Moreover, present-day liquid chromatography is mainly the high-performance liquid chromatography (HPLC), which uses a very small packing of articles. Also, HLC operates under high pressure. Therefore, the stationary phase is mainly a porous membrane or a porous monolithic layer, composed of spherical or irregular shaped particles. Meanwhile, the liquid mobile phase flows on the stationary phase under high pressure. However, there are two types of HPLC techniques according to the polarity of the mobile and stationary phases. They are the normal phase and reverse-phase liquid chromatography. Typically, in the normal phase liquid chromatography, the mobile phase is non-polar (e.g. toluene) while the stationary phase is polar (e.g. silica). On the other hand, in reverse-phase liquid chromatography, the mobile phase is polar (e.g. water-methanol mixture) while the stationary phase is non-polar (e.g. C18). However, both types of HPLC operate under room temperature.
Liquid chromatography is the other type of chromatography classified based on the physical state of the mobile phase. Significantly, its mobile phase is a liquid. For instance, the stationary phase of the liquid chromatography is solid. Therefore, the basic chromatography structure can be either a column or plane chromatography. Generally, in column chromatography, the stationary bed occurs within a tube. In contrast, in planar chromatography, the stationary phase occurs on a plane.

Figure 2: Liquid Chromatography
Moreover, present-day liquid chromatography is mainly the high-performance liquid chromatography (HPLC), which uses a very small packing of articles. Also, HLC operates under high pressure. Therefore, the stationary phase is mainly a porous membrane or a porous monolithic layer, composed of spherical or irregular shaped particles. Meanwhile, the liquid mobile phase flows on the stationary phase under high pressure. However, there are two types of HPLC techniques according to the polarity of the mobile and stationary phases. They are the normal phase and reverse-phase liquid chromatography. Typically, in the normal phase liquid chromatography, the mobile phase is non-polar (e.g. toluene) while the stationary phase is polar (e.g. silica). On the other hand, in reverse-phase liquid chromatography, the mobile phase is polar (e.g. water-methanol mixture) while the stationary phase is non-polar (e.g. C18). However, both types of HPLC operate under room temperature.
Similarities Between Gas and Liquid Chromatography
- Gas and liquid chromatography are the two types of chromatography techniques classified according to the type of mobile phase.
- Both are laboratory techniques for the separation of a mixture. Also, both are analytical separation methods.
- Generally, the mixture to be separated is dissolved in the mobile phase, which carries it through the stationary phase.
- However, the separation occurs depending on the properties of the components of the mixture, determining the variable interactions towards the mobile or stationary phase.
- Both can be column chromatography.
- Mass spectrometry (MS) is the most powerful detection method for both types of chromatography.
- Gas and liquid chromatography are the two types of chromatography techniques classified according to the type of mobile phase.
- Both are laboratory techniques for the separation of a mixture. Also, both are analytical separation methods.
- Generally, the mixture to be separated is dissolved in the mobile phase, which carries it through the stationary phase.
- However, the separation occurs depending on the properties of the components of the mixture, determining the variable interactions towards the mobile or stationary phase.
- Both can be column chromatography.
- Mass spectrometry (MS) is the most powerful detection method for both types of chromatography.
Difference Between Gas and Liquid Chromatography
Definition
Gas chromatography refers to the chromatography technique which separates and analyzes volatile compounds in the gas phase while liquid chromatography refers to the chromatography technique useful for separating ions or molecules dissolved in a solvent.
Gas chromatography refers to the chromatography technique which separates and analyzes volatile compounds in the gas phase while liquid chromatography refers to the chromatography technique useful for separating ions or molecules dissolved in a solvent.
Also Known as
Another name for gas chromatography is gas-liquid chromatography while another name for liquid chromatography is liquid-solid chromatography.
Another name for gas chromatography is gas-liquid chromatography while another name for liquid chromatography is liquid-solid chromatography.
Type of Mobile Phase
The mobile phase of gas chromatography is a gas while the mobile phase of liquid chromatography is a liquid.
The mobile phase of gas chromatography is a gas while the mobile phase of liquid chromatography is a liquid.
Examples
The mobile phase of gas chromatography is most often helium, while the mobile phase of liquid chromatography can be either polar or non-polar.
The mobile phase of gas chromatography is most often helium, while the mobile phase of liquid chromatography can be either polar or non-polar.
Mobile Phase Gradient
While the mobile phase has no gradient in gas chromatography, the mobile phase has a gradient in liquid chromatography.
While the mobile phase has no gradient in gas chromatography, the mobile phase has a gradient in liquid chromatography.
Stationary Phase
Moreover, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica.
Moreover, the stationary phase of gas chromatography is often a liquid silicone-based material while the stationary phase of liquid chromatography is mainly silica.
Chromatographic Bed Shape
Gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Gas chromatography is carried out in a column while liquid chromatography is either carried out in a column or a plane.
Columns
Long and narrow packed or capillary columns are used in gas chromatography while short and wide packed columns are used in liquid chromatography.
Long and narrow packed or capillary columns are used in gas chromatography while short and wide packed columns are used in liquid chromatography.
Sample
Components of the sample are volatile in gas chromatography, while the components of the sample are less volatile.
Components of the sample are volatile in gas chromatography, while the components of the sample are less volatile.
Chromatographic Conditions
Gas chromatography operates under high temperatures while liquid chromatography operates under high pressure.
Gas chromatography operates under high temperatures while liquid chromatography operates under high pressure.
Resolution
The resolution of gas chromatography depends on the volatility of the components of the mixture while the resolution of liquid chromatography depends on the polarity of molecules and the composition of the mobile phase.
The resolution of gas chromatography depends on the volatility of the components of the mixture while the resolution of liquid chromatography depends on the polarity of molecules and the composition of the mobile phase.
Detectors
The two main types of detectors used in gas chromatography are flame ionization detector (FID) and thermal conductivity detector (TCD) while the two main types of detectors used in liquid chromatography are ultraviolet-visible (UV/Vis) spectroscopic detector and refractive index detector (RID).
The two main types of detectors used in gas chromatography are flame ionization detector (FID) and thermal conductivity detector (TCD) while the two main types of detectors used in liquid chromatography are ultraviolet-visible (UV/Vis) spectroscopic detector and refractive index detector (RID).
Importance
Gas chromatography is mainly used in analytical chemistry while high-performance liquid chromatography is the mainly used form of liquid chromatography.
Gas chromatography is mainly used in analytical chemistry while high-performance liquid chromatography is the mainly used form of liquid chromatography.
Relative Cost
Also, gas chromatography is a low-cost technique, while liquid chromatography is a high-cost technique.
Also, gas chromatography is a low-cost technique, while liquid chromatography is a high-cost technique.
Applications
Gas chromatography is used for the separation of oils, plant pigments, pesticides, fatty acids, toxins, air samples, drug abuse testing, etc. while liquid chromatography is used for inorganic ions, polymers, sugars, nucleotides, vitamins, peptides, proteins, lipids, tetracyclines, etc.
Gas chromatography is used for the separation of oils, plant pigments, pesticides, fatty acids, toxins, air samples, drug abuse testing, etc. while liquid chromatography is used for inorganic ions, polymers, sugars, nucleotides, vitamins, peptides, proteins, lipids, tetracyclines, etc.
Conclusion
Gas chromatography is the type of chromatography, using a gas mobile phase. Generally, the mobile phase is helium. Also, the stationary phase of gas chromatography is a liquid with a silicone base. Therefore, it is a type of column chromatography. Liquid chromatography is another type of chromatography, using a liquid mobile phase, which is mainly silica. Moreover, liquid chromatography can be either column or plane chromatography. Hence, the main difference between gas and liquid chromatography is the physical state of the mobile phase.
Gas chromatography is the type of chromatography, using a gas mobile phase. Generally, the mobile phase is helium. Also, the stationary phase of gas chromatography is a liquid with a silicone base. Therefore, it is a type of column chromatography. Liquid chromatography is another type of chromatography, using a liquid mobile phase, which is mainly silica. Moreover, liquid chromatography can be either column or plane chromatography. Hence, the main difference between gas and liquid chromatography is the physical state of the mobile phase.


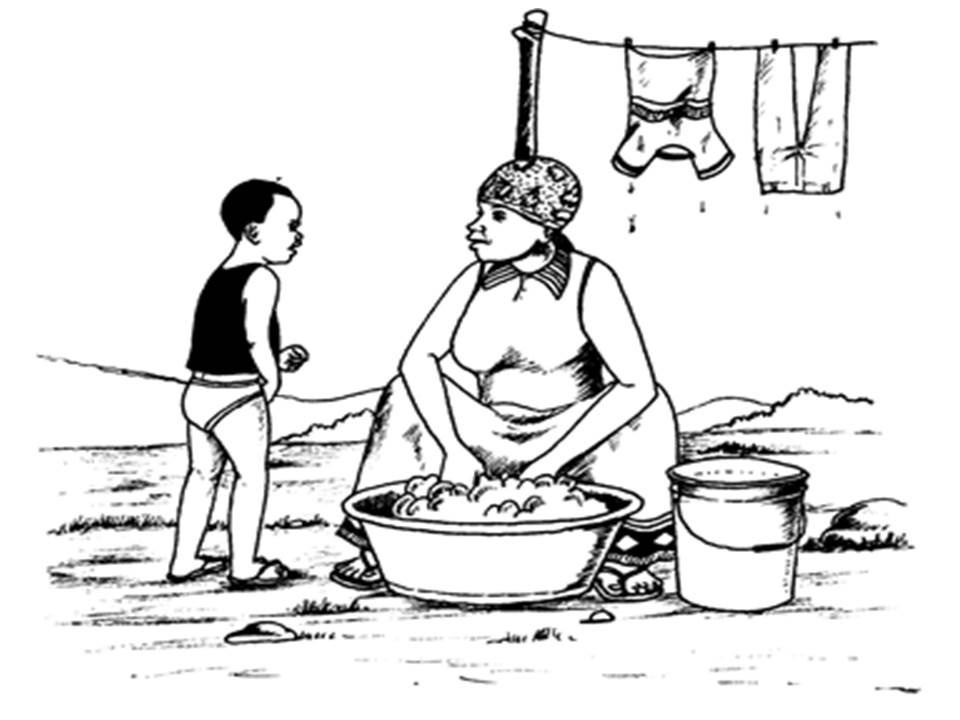
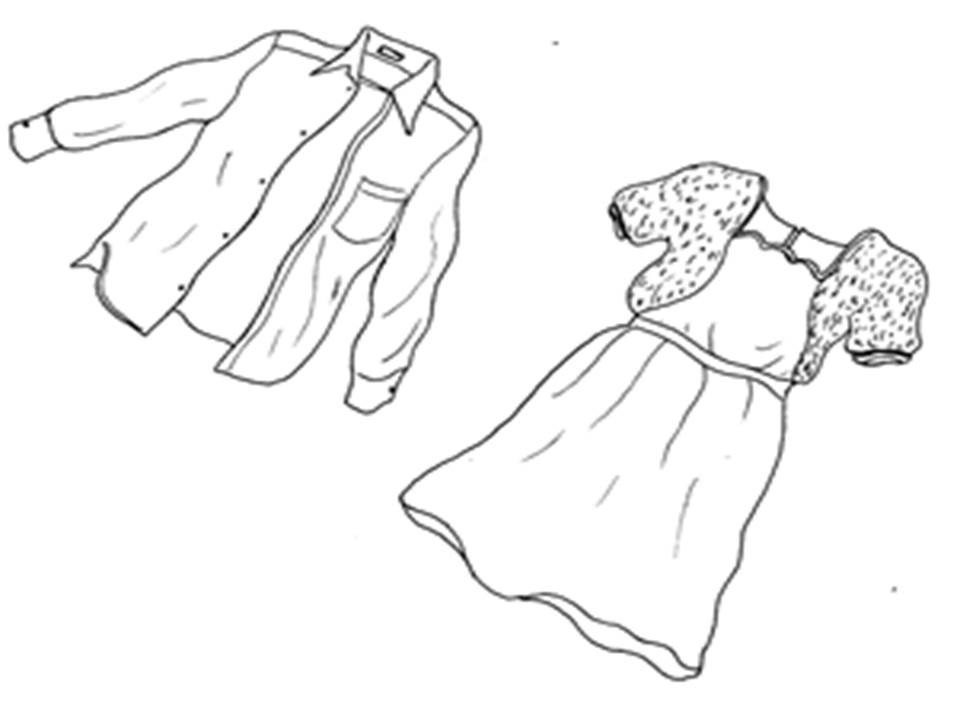



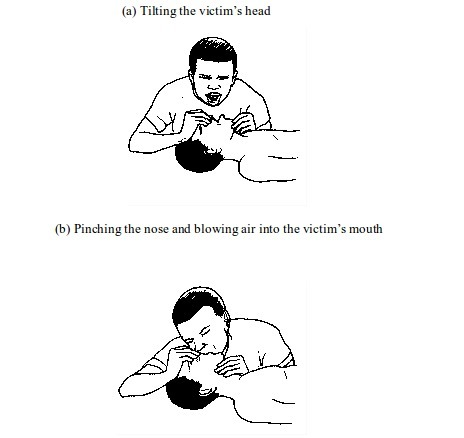
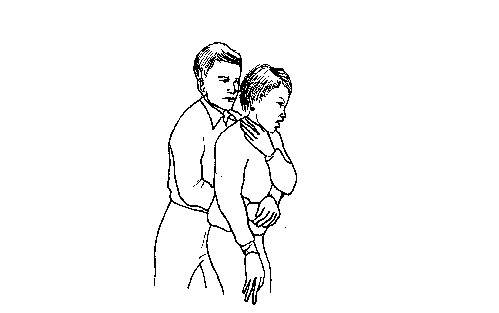
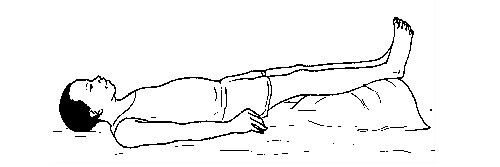

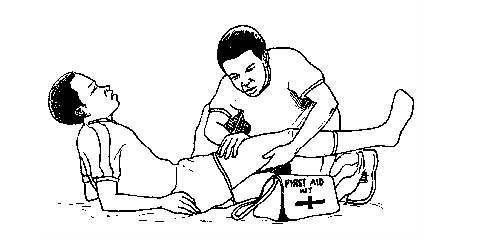
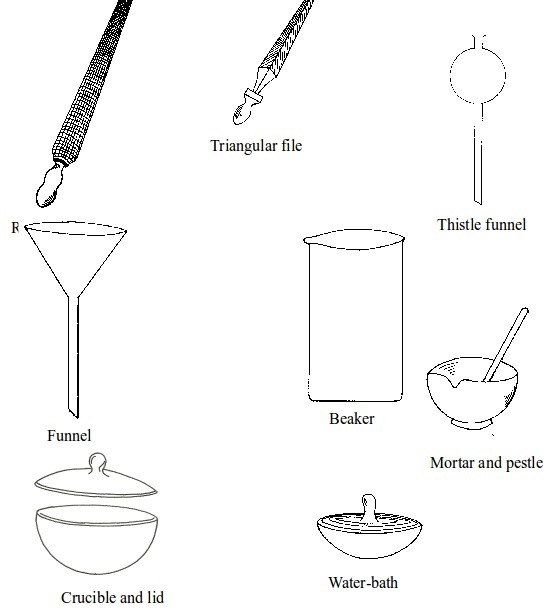
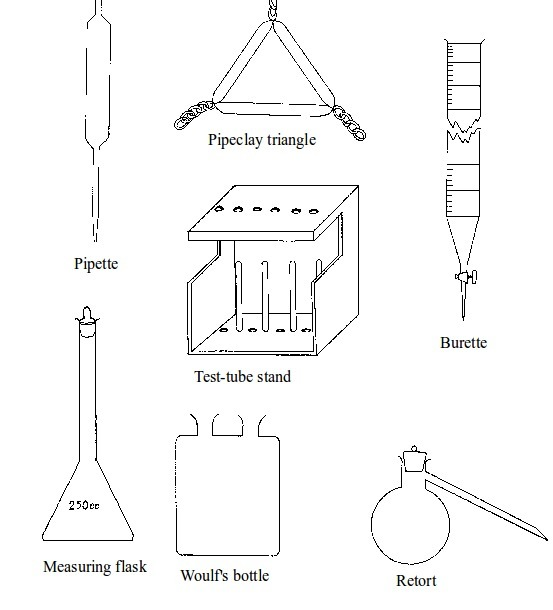
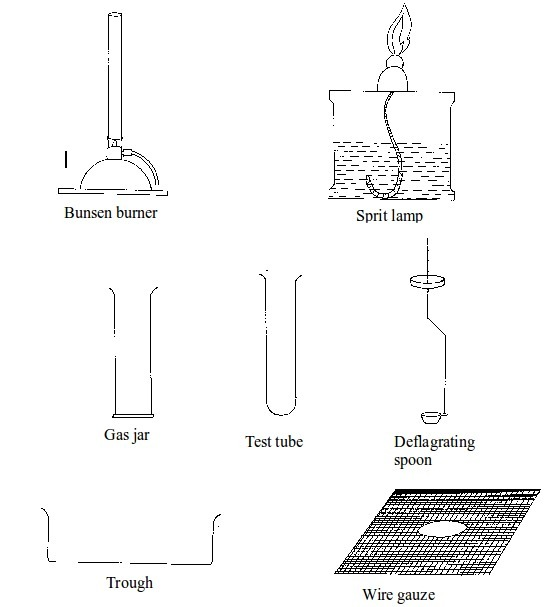
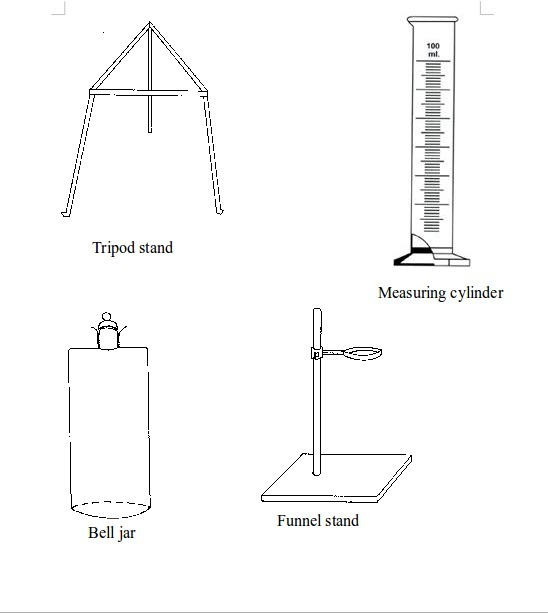
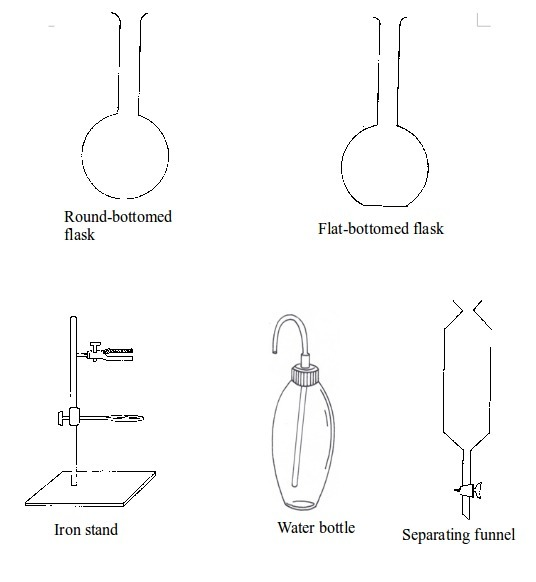
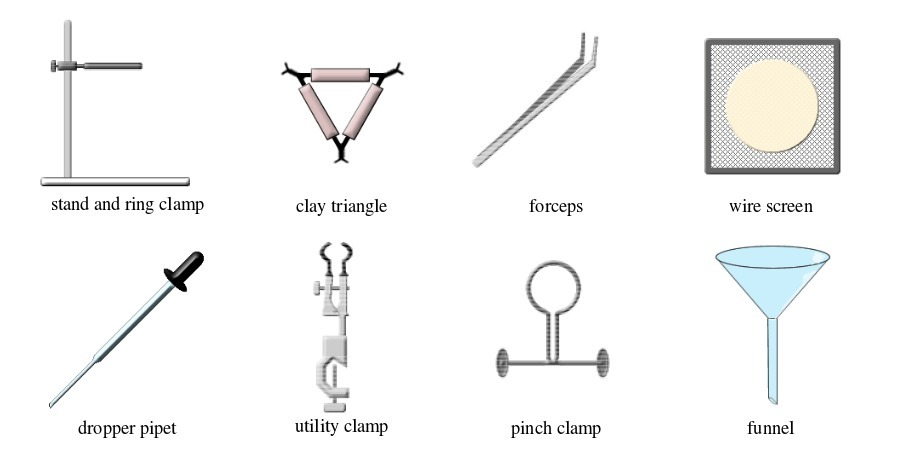

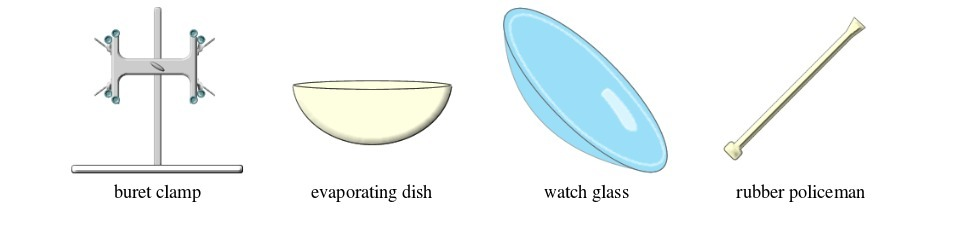
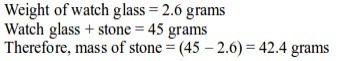
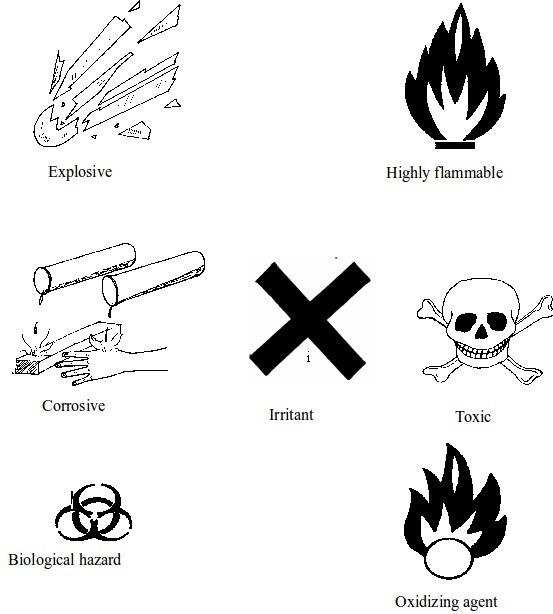
No comments:
Post a Comment